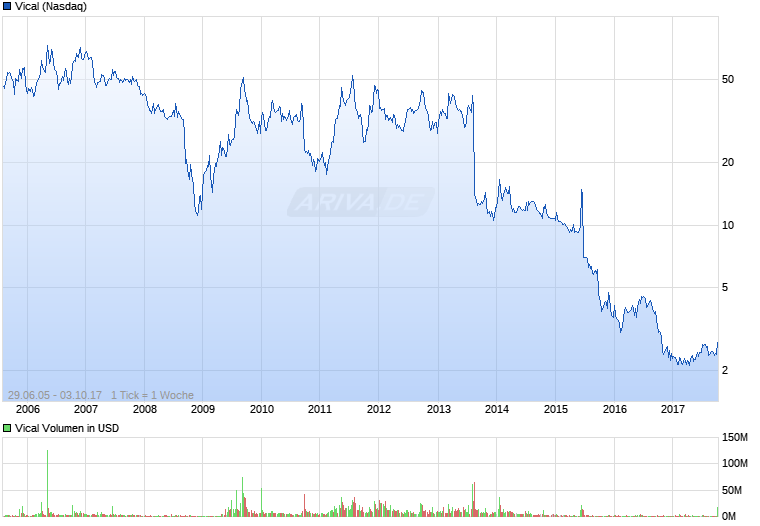
Vical - Der Neuanfang
Seite 37 von 38 Neuester Beitrag: 25.04.21 02:13 | ||||
| Eröffnet am: | 14.08.13 14:50 | von: silberfisch | Anzahl Beiträge: | 936 |
| Neuester Beitrag: | 25.04.21 02:13 | von: Ankejmrya | Leser gesamt: | 178.752 |
| Forum: | Hot-Stocks | Leser heute: | 34 | |
| Bewertet mit: | ||||
| Seite: < 1 | ... | 32 | 33 | 34 | 35 | 36 | | 38 > | ||||
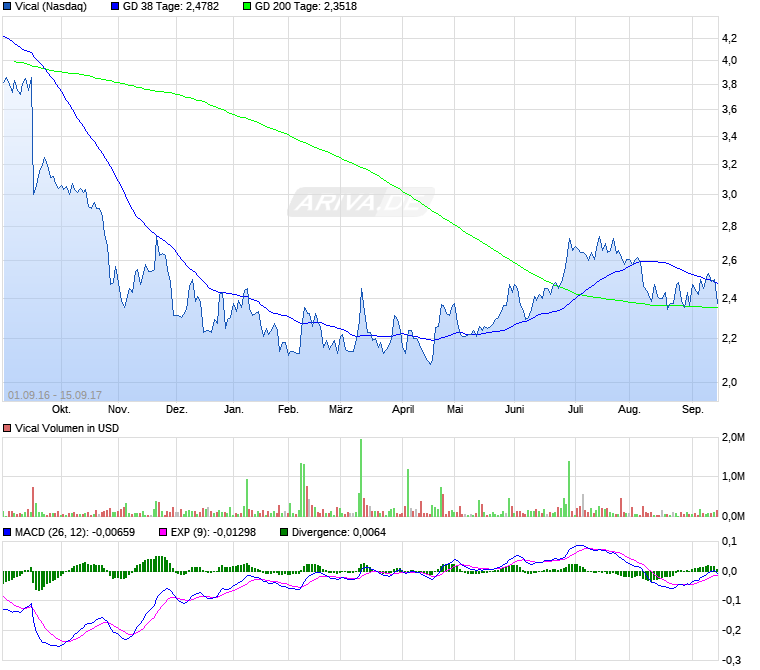
Gestern begann ein neuer Akt im Trauerspiel. Das Allzeittief bei 2,80$ wurde gerissen. Damit ist ein weiterer größerer Kursverfall sehr wahrscheinlich. Der MACD droht auch wieder bearish zu kreuzen, was den Trend weiter beschleunigen würde.
http://www.vical.com/investors/news-releases/...-Results/default.aspx
Es riecht immer mehr nach der 2-Dollar-Marke.
Geht es über 2,80$, steht 3$ auf dem Plan.
Nun also die nächste Chance. Diesmal in Form eines möglichen Doppelbodens um 2,26$ rum.
Mit Schlusskursen oberhalb 2,75$ könnte was Größeres draus werden. Alles was drunter liegt ist bedeutungslos.
http://www.vical.com/investors/news-releases/...cy-Trial/default.aspx
10/10/2017§
Astellas expects topline results in 1Q2018
SAN DIEGO, Oct. 10, 2017 (GLOBE NEWSWIRE) -- Vical Incorporated (Nasdaq:VICL) announced today that the last patient completed their final assessment during the one year follow-up period in the multinational Phase 3 registration trial of ASP0113 in hematopoietic cell transplant (HCT) recipients. The trial completed enrollment in September 2016 with a total of 515 subjects. The primary endpoint of the trial is a composite of overall mortality and cytomegalovirus (CMV) end-organ disease which will be assessed one year after transplantation. Astellas Pharma Inc. (“Astellas”) expects top-line data to be available in the first quarter of 2018.
Vicals development partner Astellas conducted the trial and has an exclusive worldwide license from Vical to develop and commercialize ASP0113. Vical developed ASP0113 through Phase 2 and continues to provide Astellas with development, regulatory and manufacturing support for the program.
“This is yet another exciting milestone for ASP0113, the first CMV vaccine to complete a pivotal Phase 3 trial,” said Vijay Samant, Vical’s Chief Executive Officer. “This has been a highly productive partnership with Astellas and both companies look forward to sharing the results of this study with the HCT community in the first quarter of 2018. Furthermore, we look forward to potentially commercializing this first-in-class, vaccine-based preventative therapy for CMV in HCT recipients.”
The Phase 3 trial enrolled patients at over 70 clinical trial sites in 11 countries throughout North America, Europe, Asia, and Australia and was designed to support full approval of ASP0113 for the prevention of CMV reactivation in CMV seropositive HCT recipients. Randomization of HCT recipients in this double-blind, placebo-controlled trial was stratified by donor-recipient relatedness and donor CMV serostatus. The trial’s primary endpoint is a composite of overall mortality and CMV end-organ disease, and secondary endpoints include time to first CMV viremia and time to CMV-specific antiviral therapy.
About CMV
CMV is a herpes virus that infects more than half of all adults in the United States by age 50, and is even more widespread in developing countries. A healthy immune system typically protects an infected person against CMV disease, but does not prevent or clear latent infection. Individuals whose immune systems are not fully functional are at high risk of CMV reactivation, potentially leading to severe illness or death. Those at greatest risk include HCT and solid organ transplant recipients, as well as infants born to mothers who first become infected during pregnancy.
About ASP0113
ASP0113 is an investigational vaccine candidate designed to prevent CMV disease and associated complications in CMV seropositive HCT recipients. ASP0113 is a bivalent DNA vaccine encoding CMV phosphoprotein 65 and glycoprotein B antigens for induction of both cellular and humoral immune responses, formulated with a proprietary poloxamer-based delivery system. ASP0113 was initially developed by Vical and has been partnered with Astellas for further development and commercialization. ASP0113 has received Orphan Drug Designation in the U.S. and Europe.
About Vical
Vical develops biopharmaceutical products for the prevention and treatment of chronic or life-threatening infectious diseases, based on its patented DNA delivery technologies and other therapeutic approaches. Additional information on Vical is available at www.vical.com.
Forward-Looking Statements
This press release contains forward-looking statements subject to risks and uncertainties that could cause actual results to differ materially from those projected. Forward-looking statements include statements about the potential uses and benefits of ASP0113, the parameters of the Phase 3 trial and timing regarding results from the Phase 3 trial. Risks and uncertainties include whether Vical's technology will be successfully applied; whether ASP0113 or any product candidates will be shown to be safe and effective in clinical trials; whether the Phase 3 trial of ASP0113 will proceed on Astellas’ expected timing or be completed, and if so, whether results will support further development or commercialization; whether ASP0113 will be successfully developed and commercialized; whether Vical or its collaborative partners will seek or gain approval to market any product candidates; whether Vical or its collaborative partners will succeed in marketing any product candidates; and additional risks set forth in the company's filings with the Securities and Exchange Commission. These forward-looking statements represent the company's judgment as of the date of this release. The company disclaims, however, any intent or obligation to update these forward-looking statements.
Contact:
Andrew Hopkins
(858) 646-1127
Website: www.vical.com
Primary Logo
Source: Vical Incorporated
11/08/2017
§
SAN DIEGO, Nov. 08, 2017 (GLOBE NEWSWIRE) -- Vical Incorporated (Nasdaq:VICL) today announced the pricing of its underwritten public offering of 14,285,714 shares of common stock or common stock equivalents at a price to the public of $1.75 per share.
H.C. Wainwright & Co., LLC is acting as the sole book-running manager for the offering.
The gross proceeds from this offering are expected to be approximately $25.0 million, before deducting underwriting discounts and commissions and estimated offering expenses payable by Vical. The offering is expected to close on or about November 10, 2017, subject to customary closing conditions. Vical has granted the underwriter a 30-day option to purchase up to an additional 2,142,857 shares of common stock in connection with the public offering, at the public offering price less underwriting discounts and commissions. All of the securities are being offered by Vical.
Registration statements on Form S-1 relating to the securities to be sold in the offering have become effective. The offering is being made only by means of a prospectus. A copy of the prospectus forming a part of the registration statement, as well as the final prospectus relating to the offering when available, may be accessed on the SEC's website located at www.sec.gov and may also be obtained by contacting H.C. Wainwright & Co., LLC, 430 Park Avenue, 4th Floor, New York, New York 10022, by calling (646) 975-6996 or emailing placements@hcwco.com.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy any of Vical’s securities. No offer, solicitation or sale will be made in any jurisdiction in which such offer, solicitation or sale is unlawful.
About Vical
Vical develops biopharmaceutical products for the prevention and treatment of chronic or life-threatening infectious diseases, based on its patented DNA delivery technologies and other therapeutic approaches.
Forward-Looking Statements
Statements in this press release regarding matters that are not historical facts, including expectations regarding the completion and timing of Vical’s proposed public offering, are forward-looking statements. These forward-looking statements are based on management's expectations and assumptions as of the date of this press release and are subject to numerous risks and uncertainties, which could cause actual results to differ materially from those expressed or implied by such statements. These risks and uncertainties include, without limitation, risks and uncertainties associated with market conditions and the satisfaction of customary closing conditions related to the proposed offering, as well as other risks and uncertainties described in Vical’s filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in Vical's Annual Report on Form 10-K for the year ended December 31, 2016 and subsequent filings with the SEC. You are encouraged to read Vical’s filings with the SEC, available at www.sec.gov, for a discussion of these and other risks and uncertainties. The forward-looking statements in this press release speak only as of the date of this press release, and Vical undertakes no obligation to update or revise any of the statements.
Contact:
Andrew Hopkins
(858) 646-1127
Website: www.vical.com
Source: Vical Incorporated
Montag, 22.01.2018 09:00 von PR Newswire
PR Newswire
TOKYO and SAN DIEGO, Jan. 22, 2018
TOKYO and SAN DIEGO, Jan. 22, 2018 /PRNewswire/ -- Astellas Pharma Inc. (TSE: 4503, President and CEO: Yoshihiko Hatanaka, "Astellas") and Vical Incorporated (NASDAQ: VICL) announced today that ASP0113, an investigational DNA vaccine being developed for cytomegalovirus (CMV)-seropositive hematopoietic stem cell transplant (HSCT) recipients, did not meet its primary or secondary endpoints in the Phase 3 HELIOS clinical trial. The vaccine was generally well tolerated, with injection-site reactions being the most commonly reported adverse event.
Astellas is a pharmaceutical company dedicated to improving the health of people around the world. (PRNewsFoto/Astellas Pharma Inc.)
"We are disappointed that the results did not demonstrate a significant improvement in overall survival and reduction in CMV end-organ disease," said Bernhardt G. Zeiher, president of Development, Astellas. "We would like to thank the patients and clinicians who participated in this important trial."
The Phase 3 trial was designed to evaluate the efficacy of ASP0113 compared with placebo in CMV-seropositive recipients undergoing an allogeneic stem cell transplant. Efficacy was assessed using a primary composite endpoint of overall mortality and CMV end-organ disease through the first year following the transplant, an endpoint which was not met. Secondary endpoints of time to first protocol-defined CMV viremia and time to first use of adjudicated CMV-specific antiviral therapy also were not met.
"The Phase 3 trial outcome is disappointing," said Vijay Samant, Vical's Chief Executive Officer. "Astellas and Vical employees, the investigators and study site personnel did an outstanding job conducting this study, but unfortunately, the vaccine was unable to provide protection against all-cause mortality in this very difficult-to-treat patient population."
The Phase 3 trial was a 1:1 randomized, double-blind, placebo-controlled study that enrolled a total of 514 CMV seropositive subjects undergoing hematopoietic stem cell transplantation. Randomization was stratified by donor-recipient relatedness and donor CMV serostatus. Subjects were followed for one year post-transplant. For more information about the ASP0113 clinical trial, please visit www.clinicaltrials.gov.
About Cytomegalovirus
CMV is a herpes virus that is estimated to infect more than half of all adults in the United States by age 50, and is even more widespread in developing countries. A healthy immune system typically protects an infected person against CMV disease, but does not prevent or clear latent infection. Individuals whose immune systems are not fully functional are at high risk of CMV reactivation, potentially leading to severe illness or death. Those at greatest risk include HCT and solid-organ transplant recipients, as well as infants born to mothers who first become infected during pregnancy.
About ASP0113
ASP0113 is an investigational vaccine candidate designed to prevent CMV disease and associated complications in CMV-seropositive HCT recipients. ASP0113 is a bivalent DNA vaccine encoding CMV phosphoprotein 65 and glycoprotein B antigens for induction of both cellular and humoral immune responses, formulated with a proprietary poloxamer-based delivery system. ASP0113 was initially developed by Vical which partnered with Astellas for further development and commercialization. ASP0113 received Orphan Drug Designation in the United States and Europe.
About Astellas
Astellas Pharma Inc., based in Tokyo, Japan, is a company dedicated to improving the health of people around the world through the provision of innovative and reliable pharmaceutical products. We focus on Urology, Oncology, Immunology, Nephrology and Neuroscience as prioritized therapeutic areas while advancing new therapeutic areas and discovery research leveraging new technologies/modalities. We are also creating new value by combining internal capabilities and external expertise in the medical/healthcare business. Astellas is on the forefront of healthcare change to turn innovative science into value for patients. For more information, please visit our website at https://www.astellas.com/en.
About Vical
Vical develops biopharmaceutical products for the prevention and treatment of chronic or life-threatening infectious diseases, based on its patented DNA delivery technologies and other therapeutic approaches. Additional information on Vical is available at www.vical.com
Astellas Cautionary Notes
In this press release, statements made with respect to current plans, estimates, strategies and beliefs and other statements that are not historical facts are forward-looking statements about the future performance of Astellas. These statements are based on management's current assumptions and beliefs in light of the information currently available to it and involve known and unknown risks and uncertainties. A number of factors could cause actual results to differ materially from those discussed in the forward-looking statements. Such factors include, but are not limited to: (i) changes in general economic conditions and in laws and regulations, relating to pharmaceutical markets, (ii) currency exchange rate fluctuations, (iii) delays in new product launches, (iv) the inability of Astellas to market existing and new products effectively, (v) the inability of Astellas to continue to effectively research and develop products accepted by customers in highly competitive markets, and (vi) infringements of Astellas' intellectual property rights by third parties.
Information about pharmaceutical products (including products currently in development) which is included in this press release is not intended to constitute an advertisement or medical advice.
Cision View original content with multimedia:http://www.prnewswire.com/news-releases/...-recipients-300585683.html
SOURCE Astellas Pharma Inc.
http://www.vical.com/investors/news-releases/...sactions/default.aspx