Generex Biotech Corp
Seite 10 von 21 Neuester Beitrag: 25.04.21 00:09 | ||||
| Eröffnet am: | 11.01.06 11:03 | von: schibi | Anzahl Beiträge: | 515 |
| Neuester Beitrag: | 25.04.21 00:09 | von: Brigittevqkfa | Leser gesamt: | 154.730 |
| Forum: | Hot-Stocks | Leser heute: | 46 | |
| Bewertet mit: | ||||
| Seite: < 1 | ... | 7 | 8 | 9 | | 11 | 12 | 13 | ... 21 > | ||||
/PRNewswire/ -- Generex Biotechnology Corporation (Nasdaq:GNBT - News) (www.generex.com) announced today a corporate update in a letter to shareholders from Interim President and Chief Executive Officer, Mark Fletcher, addressing management's strategic initiatives for growth. The letter to shareholders reads as follows:
Since I've taken the position of CEO at Generex, the management team has been working diligently to refine its comprehensive plan for the advancement of our current proprietary drug delivery and immunotherapeutic vaccine platforms as well as the Company's strategic vision for future growth. Over the past twelve months, we have seen significant advancements in both of our platform technologies and we are encouraged by our progress.
Since Generex Oral-lyn™, our proprietary buccal insulin spray product, has been the main focus by many audiences following our Company, I will begin by providing a synopsis of that program as well as providing our vision for the future of this important buccal drug delivery platform. I would, however, like to emphasize that when evaluating our Company, one should not overlook the tremendous potential of our immunotherapeutic vaccine technologies which I will also address here in greater detail.
We are currently in an ongoing Phase III clinical trial of Generex Oral-lyn™ and have reported preliminary outcomes and trends in June of this year suggesting a better "Adverse Event" profile than subjects using injectable insulin as well as no weight gain. In fiscal 2010, the safety and efficacy profile of Generex Oral-lyn™ led the Food and Drug Administration (FDA) to grant us approval for the treatment use of Generex Oral-lyn™ in patients with Type 1 or Type 2 diabetes mellitus under the FDA's "Treatment IND" program and later in that same fiscal year we were permitted to charge for the product to recover costs. Receiving that approval is an important milestone for our Company in that we are the first diabetes-related product to receive an FDA Treatment IND approval and all drugs with that type of approval have historically gone on to achieve full approval from the FDA. We have moved forward with the implementation of this innovative program by enrolling participating health practitioners after securing requisite institutional review board approvals (see www.clinicaltrials.gov for regular updates). I would also like to emphasize that while it is easy to look at some of the high profile pharmaceutical failures related to other non-injectable forms of insulin and just write off Generex Oral-lyn™ as more-of-the-same, the fact is that Generex Oral-lyn™, as opposed to inhaled insulin, does not and cannot go into the lungs. In addition, Generex Oral-lyn™ does not enter (a) the gastrointestinal tract where limited absorption and removal by the liver compromises clinical efficacy, or (b) the nasal cavity where the fragile membrane limits usefulness, or (c) through the skin where extraordinary devices are required. As the route of absorption of Generex Oral-lyn™ is through the epithelial lining of the buccal cavity, the safety and efficacy hurdles facing other forms of non-injectable insulin do not confront our product. Results from countless studies indicate a strong desire among diabetes patients for an easy and safe alternative to insulin injections.
For the future of our buccal drug delivery platform, we intend to pursue a three-pronged approach. First, we intend to complete our Phase III study of Generex Oral-lyn™, which currently has 460 patients enrolled, and then seek FDA and global approvals provided that the data remains consistent with previous data from this and other studies we have conducted. As we move through the remainder of our Phase III trial, we intend to focus our regulatory approval efforts in the United States, the European Union, and Canada. Generex Oral-lyn™ has been approved for sale in India by the Central Drugs Standard Control Organization (CDSCO), Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. We entered into a Product Licensing & Distribution Agreement with Shreya Life Sciences Pvt. Ltd. (www.shreya.co.in) pursuant to which Shreya is responsible for the marketing, distribution, and sale of Generex Oral-lyn™ in India. The terms of the Indian government approval required that a post-approval marketing surveillance study be undertaken in India. The CDSCO reviewed the protocol in respect of the study and granted permission for the commencement and prosecution of the study. The study, involving as many as 200 subjects, is well underway and is being undertaken and managed by Shreya. Upon completion of the study, the results will be submitted to the CDSCO in support of Shreya's intention to commence commercial distribution of Generex Oral-lyn™ in India.
The second prong to our strategy is to work with strategic partners to further the development and distribution of Generex Oral-lyn™ as well as other applications of our buccal drug delivery platform. Our recent long-term marketing and distribution agreement with Merck, S.A. de C.V. in Mexico for the distribution of one of our proprietary consumer/over-the-counter products, Glucose RapidSpray™ (www.glucoserapidspray.com), is just the beginning of what we believe is a tremendous commercialization opportunity for this platform. We continue to be in discussions with a number of companies with regard to potential strategic relationships in this regard, for Generex Oral-lyn™ as well as other diabetic and energy related pipeline products using the buccal delivery platform technologies.
The third prong to our strategy is to work with other pharmaceutical companies to establish the use of our proprietary liquid formulations that allow drugs typically administered by injection to be absorbed into the body by the lining of the inner mouth using our proprietary RapidMist™ device. By using RapidMist™ as a delivery method, we believe we can extend the patent life of injectable drugs near the end of their patent protection, greatly enhancing the value of those drugs.
For our immunotherapeutic vaccine platform, under development at our wholly-owned subsidiary, Antigen Express, Inc. (www.antigenexpress.com), we are confident that fiscal 2011 will be a year of great advancement in these groundbreaking efforts. We believe the vast potential of our breast, prostate, and other AE37 cancer vaccines are unmined gems for our Company. AE37 is used to generate a specific immunological response to cancers expressing the HER2 oncogene. We are in the midst of a Phase II clinical trial in patients with breast cancer and a Phase I trial in patients with ovarian cancer. In addition, we recently completed a Phase I trial in patients with prostate cancer and are making preparations to move into a Phase II trial. We anticipate our Phase II AE37 trial in breast cancer will be completed in fiscal 2011 and, provided that the study data is consistent with data available to date, we intend to work with the FDA on a plan to conduct a Phase III study for this important vaccine.
We have recently looked to augment our team of scientific advisors as we move our development programs and pipeline of products toward commercialization. We intend to take advantage of their experience and expertise to assist our scientific and management teams and provide advice and guidance in refining our science to enable us to capitalize on our Company's promising future. For Generex Oncology, we have brought in Dr. Craig Eagle, the head of the oncology therapeutic area of the global medical group for Pfizer, Inc., as an advisor to help us navigate through the regulatory approval process for AE37 as well to help us in our strategic partnership efforts within the pharmaceutical community. We have also retained the consulting services of Dr. George M. Peoples, presently the Chief of Surgical Oncology at Brooke Army Medical Center in Houston, TX, a Professor of Surgery and the Director & Principal Investigator of the Cancer Vaccine Development Program at Uniformed Services University of the Health Sciences in Bethesda, MD, and the Deputy Director of the United States Military Cancer Institute (www.usmci.org). Dr. Peoples is providing advice and assistance to us with respect to the design and implementation of a Phase III trial of the AE37 vaccine in patients with breast cancer, and acts as a liaison with the FDA regarding the clinical and regulatory development of the vaccine. We also have engaged the services of a world-renowned independent firm used by many major pharmaceutical companies for intellectual property appraisals, to provide an appraisal of our vaccine technology platform.
As I navigate our Company through this transitional period in management, I feel it is important to communicate to our shareholders that management is committed to bringing our rich intellectual property resources to commercial fruition. We continue to work closely with our business development consultants, including Seahawk Capital, which was instrumental in helping us regain our financial footing in 2009. We intend to seek out unique opportunities to generate meaningful near-term revenue streams. We are confident that, together with our board of directors, our in-house scientific team, our scientific and business development advisors, and everyone associated with our Company, we are well-positioned for, and more focused than ever on, achieving our goals.
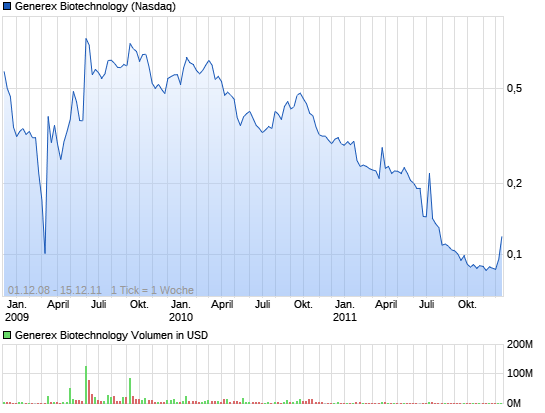
As I am sure you are aware, we are at a pivotal point in our Company's history and you are faced with an important decision. The price of our common stock is below $1 per share and we face an imminent NASDAQ delisting should our shareholders elect to vote down a proposed reverse stock split. While I fully understand the reservations you may have regarding this proposal and we will continue with our business development strategies without regard to your final decision, management believes that it is in the best interest of our Company and its shareholders to approve the proposal. We believe that by maintaining the NASDAQ listing we will be better able to attract top caliber talent to our Company as well as maintain a stronger standing in the business and financial community. We intend to communicate further on this subject prior to October 15th as we work to position Generex for the future.
In closing, I would once again like to emphasize that each and every employee and all those associated with Generex are dedicated to achieving success. As we forge ahead, I intend to make every effort to guide us toward our promising future. Generex is a company rich in intellectual properties, technologies, and talent, with tremendous growth potential, and together we will realize that potential for the benefit of our Company and its shareholders.
Quelle: www.generex.com
Generex to Release Material Announcement on October 11, 2010 | |
| October 08, 2010 | |
|
Kurs schoss gestern nach Börsenschluss um knapp 20 % hoch.
Quelle: www.generex.com
WORCESTER, Mass., Oct. 11 /PRNewswire-FirstCall/ -- Generex Biotechnology Corporation (NasdaqCM: GNBT) (www.generex.com) today announced that it has entered into a definitive agreement to acquire a majority interest (51%) in Global Medical Direct, LLC ("GMD") of Lenexa, Kansas, a nationwide Durable Medical Equipment and Pharmaceutical provider specializing in direct-to-consumer diabetes supplies and medications (www.globalmeddirect.com).
Generex Interim President & Chief Executive Officer, Mark Fletcher stated: "The acquisition of Global Medical Direct will be a significant step toward our goal of providing a cost effective distribution platform for our over-the-counter products and our existing pipeline of diabetes prescription products as they come to market. What's more, we can now reach diabetes patients, their doctors, and insurance companies with information about the FDA Treatment IND program for Generex Oral-lyn™, which they can take advantage of right now. In a single acquisition, we are bringing together the foundations for marketing, distribution, and education components needed to make Generex Oral-lyn™, upon FDA approval, the breakthrough product we believe it to be. I have been impressed by the success and management of GMD and believe this presents an ideal strategic opportunity for Generex."
GMD provides its growing roster of over 65,000 diabetes patients with an easy-to-use, cost effective, and convenient way for them to obtain their diabetes supplies and medications through their health plans or employer and government payors such as Medicare and Medicaid. GMD provides it patients with blood glucose meters, test strips, lancets, insulin, insulin pumps, and diabetes maintenance medications and supplies. GMD manages the reimbursement process for patients, dealing directly with insurers and payors. Generex sees great value in GMD's expertise in reimbursement issues in connection with the commercialization of Generex Oral-lyn™, Metformin gum, and the other products in its pipeline.
The closing of the transaction is subject to a number of terms and conditions, including the completion of a two-year financial audit, agreement upon ancillary agreements, favorable completion of due diligence, and attainment of sufficient financing by Generex. The acquisition is anticipated to close in early January, 2011. Terms of the acquisition will be included in a Form 8-K to be filed by Generex.
Based on a continuation of revenue and net income growth rates to date, GMD's management estimates its 2010 revenue will be approximately $30 million with net income exceeding $8 million, after successful double digit growth rates over the previous five years. GMD's 2009 revenue was over $22 million. The foregoing financial information is derived from management-prepared financial statements and has not yet been audited or reviewed by independent auditors.
In addition to providing a significant, profitable revenue stream to enhance Generex's bottom line, Generex believes the acquisition of GMD will bring a number of strategic benefits to accelerate revenue growth at Generex, including:
- The means to expose patients, doctors, and insurers to the existence and benefit of the Food and Drug Administration's Treatment IND program.
- The creation of a pre-launch platform with an in-house marketing, education, and distribution environment in the event of potential full FDA approval of Generex Oral-lyn™ and new Metformin products.
- Placement of Generex's own brands of glucometers, strips, and lancets into GMD's sales and marketing engine, as well as the popular Glucose RapidSpray™ product.
- A platform for Generex's diabetes related support products including soaps, vitamins, etc.
- Direct informational engagement of patients, doctors, and insurers using GMD's educational platform and call center.
- A staff experienced in dealing with the intricacies of the reimbursement policies and paperwork for diabetes-related products across U.S. state and federal governments.
- An in-house pharmacy and dispensary that dovetails with GMD's national scale shipping and distribution facility.
Approval of Reverse Stock Split
Generex management is asking its stockholders to approve a reverse stock split proposal on October 15. Management believes that a reverse stock split will put Generex in a stronger position to aggressively pursue commercialization opportunities for its buccal drug delivery and immunotherapeutic vaccine platform technologies. Based on the current price of the stock, it is anticipated that the reverse stock split ratio would not exceed 1-for-4.
If the reverse stock split is not approved, Generex's common stock will not remain listed for trading on the Nasdaq Capital Market. While the stock would then be eligible for quotation on the Over-The-Counter (OTC) Bulletin Board (OTCBB), management believes that a Nasdaq listing will afford the Company a wider variety of potentially less dilutive financing alternatives. As a development stage company, Generex must look to the capital markets to fund its on-going product development initiatives. In addition, the greater visibility and liquidity offered by a Nasdaq listing will enhance the Company's bargaining power with prospective partners and collaborators who will want comfort that Generex will have access to the capital necessary to support it contributions.
Management recognizes that many stockholders are concerned that a reverse stock split might compromise share value. However, management is not seeking the reverse stock split in a vacuum. We believe that the strength and maturity of Generex's buccal drug delivery and immunotherapeutic vaccine pipelines, together with the synergistic benefits of the proposed GMD acquisition, and financing from the proposed rights offering to stockholders, will create a solid foundation not merely for the preservation of share value but for significant growth.
Accordingly, the Board of Directors of Generex strongly urges stockholders to vote IN FAVOR of the reverse stock split proposal on October 15.
Stockholders are asked to read the details of the reverse stock split proposal set forth in the proxy solicitation materials in respect of the special meeting issued August 23, 2010 and accessible on our website at http://investor.generex.com/sec.cfm.
Should stockholders have any questions regarding the proxy voting procedures (including changing previously cast votes), please contact Legend Securities, Inc. by telephone at 877-317-7526 or via email at gnbtproxy@legendsecurities.com for US residents. Non-US residents should contact Generex directly at 800-391-6755 or contact their broker/dealer.
More on GMD:
GMD utilizes its 125+ person highly-trained, well-educated staff and call center, to maintain the ideal personal one-to-one customer relationship management environment with diabetes sufferers across the United States. Its staff establishes a continuing and ongoing dialogue with consumers to help maintain their compliance with blood glucose testing and provides them with glucometers, blood glucose test strips, lancets, insulin, and related products. GMD management expects that it will continue to recruit more than 2,500 new patients on a monthly basis. GMD is able to compile information on patient use of lancets and testing strips, thus providing a baseline for the monitoring of glucose management compliance. Glucose management compliance is of great interest to the patients' doctors and insurers and provides a reason for both groups to enroll their patients under GMD's service umbrella. Compliance in the diabetes world is essential to minimize glycemic attacks and future complications
Generex sees great value in GMD's existing diabetes educational platform. There are over 50 million pre-diabetics in the United States alone and this education strategy will allow Generex to continue to introduce and present Generex Oral-lyn™ (upon FDA approval) as well as Generex's other products to GMD's entire roster of patients as well as future consumers. Additionally, Generex, through its website and latest branding initiatives in the social media world (on Twitter, Facebook, LinkedIn, etc.), will help to serve as a platform for GMD, bringing further exposure and patient recruitment opportunities to increase its customer base.
GMD's in-house pharmacy permits the company to seamlessly handle both prescription and non-prescription drugs and handle the state-by-state intricacies of paperwork necessary for reimbursement. Generex also sees exceptional value in GMD's streamlined, and expandable packaging and shipping facility, which ships thousands of packages each month to a growing number of diabetes patients all across the United States. In anticipation of eventual marketing of Generex Oral-lyn™ when FDA approval is received, GMD's facility represents the ideal, centrally located, fully equipped national warehousing and shipping depot.
Upon completion of the acquisition, Generex intends to work closely with GMD's current management team, including Joseph Corso, Robert Shea, and Mark Franz who will remain in place to continue GMD's expansion. Joe Moscato and his team at Seahawk Capital Partners, Inc. are providing advisory services on behalf of Generex in connection with the acquisition.
Robert Shea, President & Chief Executive Officer of Global Medical Direct stated: "We are excited at the prospect of teaming up with Generex. The Generex team brings tremendous industry knowledge, expertise, and innovative technologies which will enable us to expand our market opportunities and drive both top and bottom line growth to new levels. The benefits of this combination will be immediately felt by our diabetes patients, providing them access to several new Generex OTC products, but, more importantly, they will have access to the new Generex Oral-Lyn™ buccal insulin spray product under the FDA Treatment IND program. The Generex pipeline of pharmaceutical products expected to be introduced over the next 3-5 years will position Global Medical Direct as the industry leader in the direct-to-consumer diabetes mail order segment."
About Generex Biotechnology Corporation
Generex is engaged in the research, development, and commercialization of drug delivery systems and technologies. Generex has developed a proprietary platform technology for the delivery of drugs into the human body through the oral cavity (with no deposit in the lungs). Generex's proprietary liquid formulations allow drugs typically administered by injection to be absorbed into the body by the lining of the inner mouth using Generex's proprietary RapidMist™ device. Generex's buccal insulin spray product (Generex Oral-lyn™), which has been approved in India, Lebanon, Algeria, and Ecuador for the treatment of subjects with Type-1 and Type-2 diabetes, is in Phase III clinical trials at several sites around the world. Antigen Express, Inc. is a wholly owned subsidiary of Generex. The core platform technologies of Antigen Express comprise immunotherapeutic vaccines for the treatment of malignant, infectious, allergic, and autoimmune diseases. For more information, visit the Generex website at www.generex.com or the Antigen Express website at www.antigenexpress.com. Information contained in, or accessible through, the websites of Generex or Antigen Express is not incorporated herein and is not a part of the proxy soliciting material.
Safe Harbor Statement
This release and oral statements made from time to time by Generex representatives in respect of the same subject matter may contain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These statements can be identified by introductory words such as "expects," "plans," "intends," "believes," "will," "estimates," "forecasts," "projects," or words of similar meaning, and by the fact that they do not relate strictly to historical or current facts. Forward-looking statements frequently are used in discussing potential product applications, potential collaborations, product development activities, clinical studies, regulatory submissions and approvals, and similar operating matters. Many factors may cause actual results to differ from forward-looking statements, including inaccurate assumptions and a broad variety of risks and uncertainties, some of which are known and others of which are not. Such risks and uncertainties include the risks that: (1) Generex will not obtain the stockholder approval of the reverse stock split; (2) the reverse stock split, if implemented, will fail to have the desired effect of sufficiently raising the common stock price to meet The NASDAQ Capital Market's $1.00 minimum bid price requirement for continued listing of Generex's stock; (3) the conditions to the closing of the acquisition of Global Medical Direct, LLC will not be satisfied; and (4) the anticipated benefits of the proposed right offering to stockholders will not be realized. Known risks and uncertainties also include those identified from time to time in the reports filed by Generex with the Securities and Exchange Commission, which should be considered together with any forward-looking statement. No forward-looking statement is a guarantee of future results or events, and one should avoid placing undue reliance on such statements. Generex undertakes no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Generex cannot be sure when or if it will be permitted by regulatory agencies to undertake additional clinical trials or to commence any particular phase of clinical trials. Because of this, statements regarding the expected timing of clinical trials or ultimate regulatory approval cannot be regarded as actual predictions of when Generex will obtain regulatory approval for any "phase" of clinical trials or when it will obtain ultimate regulatory approval by a particular regulatory agency. Generex claims the protection of the safe harbor for forward-looking statements that is contained in the Private Securities Litigation Reform Act.
Generex Contacts:
Should stockholders have any questions regarding the corporate events described in this release or have questions regarding the proxy voting procedures (including changing previously cast votes), please contact Legend Securities, Inc. by telephone at 877-317-7526 or via email at gnbtproxy@legendsecurities.com for US residents. Non-US residents should contact Generex directly at 800-391-6755 or contact their broker/dealer.
Quelle: yahoo.com
Press Release Source: Generex Biotechnology Corporation On Monday October 11, 2010, 10:31 am
WORCESTER, Mass., Oct. 11 /PRNewswire/ -- Generex Biotechnology Corporation (Nasdaq:GNBT - News) (www.generex.com) today announced that it plans to initiate a rights offering of common stock and warrants to its existing stockholders in the event that the stockholders approve a reverse stock split at the reconvened special meeting of stockholders scheduled for 10 a.m. EDT on Friday, October 15, 2010. The amount of the offering has not been determined, but is anticipated to permit an aggregate investment of at least $25,000,000. Certain warrant holders also will have the right to participate in the offering.
Generex is preparing to file a registration statement with the Securities and Exchange Commission forthwith following stockholder approval of a reverse stock split. Generex expects the commencement of the offering and the distribution of rights to occur promptly following effectiveness of the registration statement. Generex expects to use the proceeds from the rights offering primarily to fund its on-going research & development and product commercialization initiatives and the proposed acquisition of Global Medical Direct, LLC.
The record date for the distribution of the rights and the dates for both the subscription period and the expiration of the rights offering will be included in the final prospectus. Under the proposed terms of the rights offering, Generex would distribute one right to each holder of record of every share of its common stock that is held on the record date. Each transferable right will entitle the stockholder to purchase one unit at a subscription price to be determined prior to the effective date of the registration statement. Each unit will consist of one share of common stock and two warrants to purchase additional shares of common stock.
This press release does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities.
Forward-Looking Statements
This press release includes statements about future economic performance, finances, expectations, plans and prospects of Generex that constitute forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements are based on Generex's current plans, estimates and expectations. Some forward-looking statements may be identified by use of terms such as "anticipate," "expect," "plan," "may," "should," "could," "will," "continue," "estimate," and similar words, terms or statements of a future or forward-looking nature. Such forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those expressed in or suggested by such statements. Risks and uncertainties relating to the proposed offering include the risks that: (1) Generex will not obtain the stockholder approval of the reverse stock split; (2) the reverse stock split, if implemented, will fail to have the desired effect of sufficiently raising the common stock price to meet The Nasdaq Capital Market's $1.00 minimum bid price requirement for continued listing of Generex's stock; and (3) the anticipated benefits of the right offering will not be realized. No forward-looking statement is a guarantee of future results or events, and one should avoid placing undue reliance on such statements. Generex undertakes no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Generex claims the protection of the safe harbor for forward-looking statements that is contained in the Private Securities Litigation Reform Act.
Quelle: generex.com
Generex Biotechnology Corporation (NASDAQ: GNBT) announced Tuesday that on October 19, 2010 the NASDAQ Listing Qualifications Panel notified the Company that the Panel has determined to delist the Company's common stock from the NASDAQ Capital Market and will suspend trading of the stock effective at the open of trading on Thursday, October 21, 2010 as a consequence of the Company's non-compliance with NASDAQ's minimum $1.00 per share closing bid price requirement. Shares of the company sank more than 19% in after-hours trading.
www.prnewswire.com/news-releases/...ay-october-21-2010-105292418.html
Oder liegt es daran , weil hier sich schon grosse Firmen wie Pfizer oder auch MannKind
die Finger verbrand haben.
Möglicher Milliardenmarkt
Mit Oral-lyn zielt Generex auf einen potenziellen Milliardenmarkt. Allein in Deutschland leiden Expertenschätzungen zufolge etwa 5,5 Millionen Menschen an den verschiedenen Typen der im Volksmund Zuckerkrankheit genannten Diabetes. Die Patienten sind auf die regelmäßige Zufuhr von Insulin angewiesen, die Verabreichung durch eine schmerzhafte Injektion stellt aber für viele Betroffenen eine große Belastung dar.
Übernahmekandidat
Sollten sich die bisherigen Ergebnisse auch am Ende der Studie bestätigen, könnte Generex also ein großer Wurf gelingen – und dürfte damit unweigerlich das Interesse größerer Pharma-Unternehmen auf sich ziehen. Die zahlen die aktuelle Börsenbewertung von Generex – etwa 55 Millionen Dollar – aus der Portokasse. Anleger mit extrem hoher Risikotoleranz können auf ein solches Szenario spekulieren und sich einige Stücke ins Depot legen.
http://www.deraktionaer.de/aktien-usa/...x--kursexplosion-9723759.htm
Das Produkt „Oral-lyn“ in Phase III wie aber auch Krebsmedikament „AE37“ in Phase II
haben Blockbusterpotential !
Ich denke, es liegt aber auch daran, dass solche Firmen wie Genta oder auch Novelos Therapeutics
die Anleger stark getäuscht bzw. enttäuscht hatten. ( mich eingeschlossen )
Ein Totalverlust kann auch hier natürlich nicht ausgeschlossen werden.
Zitat :
Anleger mit extrem hoher Risikotoleranz können auf ein solches Szenario spekulieren und sich einige Stücke ins Depot legen! ( und da stand der Kurs bei ca. 50 Cent )
http://www.deraktionaer.de/aktien-usa/...x--kursexplosion-9723759.htm
The number of outstanding shares of the registrant's common stock, par value $.001, was 290,671,282 as of March 10, 2011
Alles nach zulesen unter :
http://yahoo.brand.edgar-online.com/...fault.aspx%253fcik%253d1059784
Einiges auf deutsch übersetzen lassen
Generex Mündlich-lyn™ Genehmigungen durch die zuständige Aufsichtsbehörde und klinische Studien Bis jetzt haben wir Genehmigung durch die zuständige Aufsichtsbehörde in Ecuador, Indien, der Libanon empfangen und Algerien für das Handelsmarketing und den Verkauf von Generex Mündlich-lyn™, obgleich wie pro die Bedingungen der Genehmigung durch die zuständige Aufsichtsbehörde in Indien, eine lokale klinische Studie geleitet werden muss, bevor das Produkt für Handelsverkauf in diesem Land angeboten werden kann. Im März 2008 leiteten wir klinische Studien der Phase III für dieses Produkt in den US mit der ersten geduldigen Siebung für solche Versuche an einem klinischen Studienaufstellungsort in Texas ein. Die geduldige Siebung an anderen teilnehmenden klinischen Aufstellungsorten in den US und im Kanada läuft. Bis jetzt über 450 Patienten sind in 70 klinischen Aufstellungsorten um die Welt, einschließlich Aufstellungsorte in den Vereinigten Staaten, im Kanada, im Ecuador, im Bulgarien, im Polen, im Rumänien, im Russland und in der Ukraine eingeschrieben worden. Im November 2008 reichten wir unser Produktdossier beim Gesundheitsministerium in Damaskus, Syrien durch Generex MENA, unsere Zweigniederlassung in Dubai ein. Das Dossier umfaßt Mund-lyn™ Generex. Wir reichten auch eine Akte ein, um unsere eigenen im Freiverkehr gehandelten Produkte, einschließlich Glukose RapidSpray™, 7-tägiges Diät-Hilfsmittel Spray™ (vermarktet wie sich Sehnen-Nx™ in den Vereinigten Staaten und im Kanada) und BaBOOM zu registrieren! ™ Energie-Spray. Das syrische Gesundheitsministerium hat das Dossier für Mund-lyn™ Generex wiederholt und hat eine viermonatliche Inlandklinische Studie genehmigt, für die geduldige Verstärkung begonnen hat und die Versuch erwartet wird, um früh in Kalenderjahr 2011 anzufangen. Nach erfolgreicher Beendigung dieses Versuches, nehmen wir vorweg, dass Genehmigung durch die zuständige Aufsichtsbehörde kurz danach folgt. Wir nehmen nicht die bedeutenden von vorweg dieser Zustimmung im 2011 Finanzjahr erkannt zu werden Einkommen. Wir haben auch regelnde Dossiers für Generex eingereicht, das in einigen anderen Ländern einschließlich Bangladesh, Kenia, Yemen, den Irak, den Iran, Libyen und Sudan Mündlichist. Während wir glauben, genehmigen diese Länder schließlich unser Produkt für Handelsverkauf, könnte es einige Zeit frühestens sein, bevor diese Zustimmungen werden empfangen und da so wir die Anerkennung keiner Einkommen für diese Jurisdiktionen bis das letzte Teil des 2011 Kalenderjahres vorwegnehmen. Spezieller Zugangs-Programme Im Oktober 2009 empfingen wir Zustimmung vom US-Behörde zur Überwachung von Nahrungs- und Arzneimitteln (die „FDA ") um aufzuladen, um Kosten für den Behandlunggebrauch Generex Mündlich-lyn™ bei Patienten mit Typ 1 zu decken oder - 2 Diabetes mellitus im Behandlung-neuen Drogeuntersuchungsprogramm der FDA zu schreiben, das für frühen Zugang zu den Untersuchungsbehandlungen für die lebensbedrohenden oder anders ernsten Bedingungen zur Verfügung stellt. 14 Wir empfingen eine Ermächtigung des spezieller Zugangs-Programms („SAP ") von der Gesundheit Kanada für eine Patient-spezifische, Arzt-überwachte Behandlung von Typ 1diabetes mit Generex, das im April 2008 Mündlichist. SAP bietet Zugang zu nicht-vermarkteten Drogen für die Praktiker, die Patienten mit den ernsten oder lebensbedrohenden Bedingungen behandeln, wenn herkömmliche Therapien ausgefallen sind, sind nicht vorhanden oder unpassend. Wir empfingen eine ähnliche Ermächtigung von den Gesundheitsämtern in den Niederlanden im September 2008. Wir fahren fort, unsere SAP-Teilnahme an den zusätzlichen Ländern um die Welt zu erweitern. Marketing Wir haben Genehmigen und Verteilungsverträge mit einigen multinationalen Verteilern, uns mit dem Prozess von Genehmigung durch die zuständige Aufsichtsbehörde für die Ausrichtung, das Marketing, die Verteilung schlossen, und den Verkauf von Generex weltweit gewinnen zu unterstützen Mündlich-lyn™ in den Ländern und umfaßt: · Shreya Biowissenschaften Pvt. Ltd. für Indien, Pakistan, Bangladesh, Nepal, Bhutan, Sri Lanka und Myanmar; · Adcock Ingram begrenzt und Ltd. der Adcock-Ingram Gesundheitspflege-(PTY) für Südafrika, Lesotho, Swasiland, Botswana, Namibia, Mosambik und Zimbabwe; · E& V Alca Distribution Corp. für Albanien, Montenegro und Kosovo; · Medrey S.A.L. (früher MedGen Corp.) und Benta S.A.L. für den Libanon; · SciGen, Ltd. für China, Hong Kong, Indonesien, Malaysia, die Philippinen, Singapur, Thailand und Vietnam; · Pharmaris Perus S.A.C. für Peru; · MediPharma SA für Argentinien · PMG S.A. für Chile; und · Dong gesungenes Pharm. Co. Ltd. für Südkorea. Im August 2008 nahmen wir an einem Produkt genehmigend und Verteilungsvereinbarung mit Dong gesungenen Pharm Co. Ltd. für den Import, das Marketing teil, die Verteilung und den Verkauf von Generex Mündlich-lyn™ in Südkorea. Unter der siebenjährigen Vereinbarung Dong-Gesungen hat eine ausschliessliche Lizenz. Pro die Vertragsbedingungen zahlten sie uns eine non-refundable Lizenzgebühr USD-$500.000 nach Durchführung und werden uns eine non-refundable Lizenzgebühr USD-$500.000 zu einem Zeitpunkt zahlen, wie Regierungszustimmung für den Import, das Marketing, die Verteilung und den Verkauf des Produktes in Südkorea erreicht wird. Unter dieser Vereinbarung sind wir für das Verschaffen solcher Regierungszustimmung verantwortlich. Zusätzlich wenn sie seinen ersten Kaufauftrag erteilt, Dong-Gesungen bildet uns in der Menge von USD $500.000 eine Vorauszahlung, die gegen ProduktKaufaufträge angewandt sind. Unser Generex MENA Büro, gelegen in der Dubai-Gesundheitspflege-Stadt, hat Unterordnungen des Generex Mund-Lyn™ Dossiers mit Aufsichtsbehörden in dem Mittlere Osten-und Nordafrika archiviert und hat ein Verteilungsnetz innen über 20 Ländern eingerichtet. Dieses Verteilungsnetz ist für die Weiterverfolgung mit den Dossiers verantwortlich, die in ihren spezifischen Regionen eingereicht werden und ist auch aktiv gewesen, verteilend kaufend und die Süßigkeitenlinie der Firma der Produkte. In Indien ist ein Vermarktungsplan bereits durch ShreyaBiowissenschaften Pvt eingereicht worden. Ltd., zu Generex auf der Marketingstrategie für die Verteilung von MundRecosulin™, das das eingetragene Warenzeichen ist, unter dem Shreya Generex vermarktet, das innerhalb Indiens Mündlichist. Pro die Anforderungen der Genehmigung durch die zuständige Aufsichtsbehörde in Indien, muss eine klinische Inlandstudie in Indien mit MundRecosulin™ abgeschlossen werden, bevor Handelsverkäufe beginnen können.
Generex Biotechnology Corp. expected to report Q3 2011 results on June 9, 2011. This event was calculated by Capital IQ (Created on March 12, 2011).
03/12/2011
Schlüsselentwicklungen für GENEREX BIOTECHNOLOGY CORP (GNBT) Generex Biotechnology Corp. erwartete, Resultate Q3 2011 zu melden am 9. Juni 2011. Dieses Ereignis wurde durch Haupt-IQ berechnet (am 12. März 2011 verursacht). 03/12/2011 Generex Biotechnology Corp. erwartete, Resultate Q3 2011 zu melden am 9. Juni 2011. Dieses Ereignis wurde durch Haupt-IQ berechnet (am 12. März 2011 verursacht).
Bis zum Sommer wird die Kohle wohl noch reichen ,dann wirds eng !
http://ycharts.com/financials/GNBT/quarterly_cash_flow
Was mein Ihr dazu ?
Hörte sich so schön an mit einem Insulin-Spray... aber warum haben die großen Pharma-Konzerne da nicht schon längst zugeschlagen?
http://pipeline.corante.com/archives/2010/04/07/...pray_just_hype.php
WORCESTER, Mass. and TORONTO, Dec. 8, 2011 /PRNewswire/ -- Generex Biotechnology Corporation (OTCBB: GNBT.OB) today announced that positive interim Phase 2 clinical data from its ongoing study of a novel Ii-Key Hybrid-based HER-2/neu Peptide Vaccine (AE37) in HER-2 expressing breast cancer patients were presented at the 34th Annual CTRC-AACR San Antonio Breast Cancer Symposium (SABCS) in San Antonio, Texas. The AE37 vaccine is being developed by its wholly-owned subsidiary, Antigen Express, Inc.
(Logo: http://photos.prnewswire.com/prnh/20110106/NY25057LOGO-b)
"We are encouraged by the positive interim results in disease-free survival demonstrated in the randomized Phase 2 study with AE37 vaccine, especially in patients with low HER2 expression that are not currently eligible for Herceptin® (trastuzumab; Roche-Genentech)," said Dr. Eric von Hofe, Ph.D., President of Antigen Express. "While the number of patients with recurrent breast cancer is still too low to demonstrate statistical significance in this ongoing study, we project a sufficient number of events in 2012 and expect to report final results during this period."
The results were presented at SABC by COL George E. Peoples, MD's Cancer Vaccine Development Program on December 7th. "Women with breast cancers expressing low levels of HER2 do not benefit from targeted HER2 therapies that are currently available," said COL Peoples, a leading researcher in adjuvant breast cancer vaccine development. "Our research is focused on reducing the recurrence of cancer using a woman's own immune system to fight her disease, including breast cancers that express low levels of HER2. The AE37 vaccine is based on over 5 years of research and continues to show promise in a well-designed and ongoing randomized Phase 2 clinical trial that if positive, will allow rapid transition to Phase 3."
AE37 is the subject of an ongoing, controlled, randomized, and single-blinded Phase 2 clinical study in human epidermal growth factor receptor 2 (HER2) expressing patients with either node positive or high-risk node-negative breast cancer. Patients are randomized to receive AE37 plus granulocyte-macrophage colony-stimulating factor (GM-CSF) or GM-CSF alone (control). The primary endpoint is a reduction in cancer relapse after two years.
There are currently over 250 patients enrolled in the Phase 2 study. Kaplan-Meier projections of updated data presented at SABCS ("An Update of a Phase II Trial of the HER2 Peptide AE37 Vaccine in Breast Cancer Patients to Prevent Recurrence," abstract #PT1-13-01) demonstrate that disease-free survival in the low HER2 expressing patients was 88.6% in the treated group (n=53) versus 71.9% in the control arm (n=78) at a median follow-up of 22 months.
Patients treated with vaccine also exhibited a statistically significant increase in positive immune reactions to a test dose of HER2 (AE36 (HER2:776-790)) protein including maintenance of positive immune response up to 12 months post-vaccination while there have been no changes in immune responses for control patients.
The Company is assessing the data for potential opportunity to move forward with a Phase 3 clinical development program following an End-of-Phase 2 meeting with the U.S. Food and Drug Administration (FDA) for AE37, which Antigen Express believes, if confirmed, could occur in the first half of 2012.
About AE37 and Ii-Key Hybrid Platform Technology
Antigen Express is a platform technology and product-based company developing proprietary vaccine formulations for large, unmet medical needs. The Company's Ii-Key Hybrid technology platform entails the modification of fragments of antigens to increase their potency in stimulating critical members of the immune response, known as CD4+ T helper cells. Incorporating the Ii-Key modification along with tumor-associated antigens can greatly enhance the immune system's ability to recognize and destroy cancer cells bearing any of the targeted antigens as well as increasing immunological memory.
The first product candidate utilizing the Company's novel Ii-Key Hybrid technology platform is a HER-2/neu Peptide Vaccine (AE37). This "off-the-shelf" cancer immunotherapy product candidate is easier and less costly to produce than comparable cell-based approaches. AE37 is derived from a peptide fragment of the human epidermal growth factor receptor 2 (HER2) oncoprotein, which is expressed in a variety of tumors including 75-80% of breast cancers as well as a high percentage of prostate, ovarian and other cancers. AE37 represents the only HER2-based peptide vaccine currently being studied in a randomized trial and its use is not restricted to patients with a particular type of human leukocyte antigen (HLA) peptide.
About Breast Cancer
According to the American Cancer Society, more than 232,000 women will be diagnosed with breast cancer, and nearly 40,000 will die from the disease, in 2011. For women whose cancer tests positive for increased quantities of the HER2, approved targeted therapies include trastuzumab (Herceptin®; Roche-Genentech). However, only 25% of breast cancer patients have HER2 levels high enough to be eligible for Herceptin. AE37 is positioned initially as an adjuvant therapy for at least 50% of breast cancer patients; i.e., those with low-to-intermediate levels of HER2 expression.
About Generex Biotechnology Corporation
Generex is engaged in the research, development, and commercialization of drug delivery systems and technologies. Generex has developed a proprietary platform technology for the delivery of drugs into the human body through the oral cavity (with no deposit in the lungs). The Company's proprietary liquid formulations allow drugs typically administered by injection to be absorbed into the body by the lining of the inner mouth using the Company's proprietary RapidMist™ device. Antigen Express, Inc. is a wholly owned subsidiary of Generex. The core platform technologies of Antigen Express comprise immunotherapeutic vaccines for the treatment of malignant, infectious, allergic, and autoimmune diseases. Antigen Express has pioneered the use of specific CD4+ T-helper stimulation in immunotherapy. One of its platform technologies relies on inhibition of expression of the Ii protein. Antigen Express scientists, and others, have shown clearly that suppression of expression of the Ii protein in cancer cells allows for potent stimulation of T-helper cells and prevents the further growth of cancer cells. For more information, visit the Generex website at www.generex.com or the Antigen Express website at www.antigenexpress.com.
Cautionary Note Regarding Forward-Looking Statements
This release and oral statements made from time to time by Generex representatives in respect of the same subject matter may contain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These statements can be identified by introductory words such as "expects," "plan," "believes," "will," "achieve," "anticipate," "would," "should," "subject to" or words of similar meaning, and by the fact that they do not relate strictly to historical or current facts. Forward-looking statements frequently are used in discussing potential product applications, potential collaborations, product development activities, clinical studies, regulatory submissions and approvals, and similar operating matters. Many factors may cause actual results to differ from forward-looking statements, including inaccurate assumptions and a broad variety of risks and uncertainties, some of which are known and others of which are not. Known risks and uncertainties include those identified from time to time in the reports filed by Generex with the Securities and Exchange Commission, which should be considered together with any forward-looking statement. No forward-looking statement is a guarantee of future results or events, and one should avoid placing undue reliance on such statements. Generex undertakes no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Generex cannot be sure when or if it will be permitted by regulatory agencies to undertake additional clinical trials or to commence any particular phase of clinical trials. Because of this, statements regarding the expected timing of clinical trials or ultimate regulatory approval cannot be regarded as actual predictions of when Generex will obtain regulatory approval for any "phase" of clinical trials or when it will obtain ultimate regulatory approval by a particular regulatory agency. Generex claims the protection of the safe harbor for forward-looking statements that is contained in the Private Securities Litigation Reform Act.
Headlines
Filter Headlines- Breast Cancer Vaccine Being Developed by Generex Subsidiary Antigen Express to be Featured on Bloomberg TelevisionPR Newswire(Wed, Dec 14)
- GENEREX BIOTECHNOLOGY CORP Files SEC form 10-Q, Quarterly ReportEDGAR Online(Mon, Dec 12)
- Generex Biotech and Avanir Pharmaceuticals Look to Rally in 2012Marketwire(Mon, Dec 12)
- Breast Cancer Vaccine Reduces Cancer Recurrence in Women: Interim Results From Phase 2 Clinical Trial Trend Toward AE37 Vaccine Benefit; Results Presented at SABCSPR Newswire(Thu, Dec 8)
Breast Cancer Vaccine Being Developed by Generex Subsidiary Antigen Express to be Featured on Bloomberg Television
Vaccine Also Referenced in Newsweek Magazine
WORCESTER, Mass. and TORONTO , Dec. 14, 2011 /PRNewswire/ -- Generex Biotechnology Corporation (OTCBB: GNBT.OB) today announced that Bloomberg Television will air a segment on the immunotherapeutic breast cancer vaccine being developed by its wholly-owned subsidiary, Antigen Express, Inc.
The story, expected to air December 19, 2011 , will cover the Company's novel Ii-Key Hybrid-based HER-2/neu Peptide Vaccine (AE37), which is the subject of an ongoing Phase 2 clinical study in patients with HER-2 expressing breast cancer. The segment will be featured in Bloomberg's "Innovators"
segment, which focuses on world-changing technologies and innovations.
Positive interim results from the ongoing Phase 2 study of AE37 were presented at the 34th Annual CTRC-AACR San Antonio Breast Cancer Symposium (SABCS) earlier this month. Final Phase 2 results are expected in 2012.
The segment will include an interview by Bloomberg reporter Cali Carlin of Dr. Eric von Hofe , Ph.D., President of Antigen Express, and COL George E. Peoples , MD, FACS, Director, Cancer Vaccine Development Program.
"Development of the Antigen Express immunotherapeutic vaccine technologies has progressed to the point where people are beginning to take notice," commented Mark Fletcher , President & Chief Executive Officer of Generex. "This Bloomberg piece follows on the heels of the SABCS presentation and the%