Cytori Therapeutics
Jun 14, 2010
http://ir.cytoritx.com/InvestorRelations/...tail.cfm?ReleaseID=479210
Cytori Gains Stem Cell Device Approval in Europe for Breast Cancer Reconstruction and Soft Tissue Repair
Press Release Source: Cytori Therapeutics On Tuesday July 27, 2010, 8:00 am
SAN DIEGO--(BUSINESS WIRE)--Cytori Therapeutics (NASDAQ: CYTX - News) received expanded European approval (CE Mark) for its Celution® System, a medical device that extracts and separates stem and regenerative cells from a patient’s own fat tissue. The new indications include key medical applications such as breast reconstruction, repair of soft tissue defects, as well as the facilitation of healing certain types of wounds, such as those resulting from Crohn’s disease. This broadens Cytori’s ability to offer the Celution® System to patients and hospitals and further supports the company’s efforts to gain treatment reimbursement.
Cell-enriched reconstruction is an innovative single treatment procedure that addresses a variety of soft tissue defects and conditions, including the unmet physical and psychological needs created by partial mastectomy. This approach uses a woman’s own fat tissue combined with her own naturally available adipose-derived stem and regenerative cells to form a ‘cell-enriched’ fat graft, which is used to reconstruct the affected breast or other soft tissue defects.
Clinical data from Cytori’s RESTORE 2 breast reconstruction trial in Europe and other wound repair clinical studies were used to support the expanded indications. The previously approved indications for use on the Celution® System remain active and unchanged. The new indications specifically cover the Celution® System for the digestion of adipose tissue to extract, wash and concentrate a patient’s own stem cells and other associated cells for use in the following procedures:
•Preparation and implantation of autologous cell-enriched fat grafts for cell-enriched breast reconstruction, the first approval for a regenerative technology for this application, as well as for other soft tissue defects;
•Preparation and implantation of autologous cell-enriched fat grafts for elective procedures, such as breast and buttocks augmentation and facial applications;
•The delivery of the Celution® System cellular output to facilitate healing of rectal and vaginal fistulas (wounds) resulting from Crohn’s disease, the first stem cell-based approval in Europe for this condition.
“The expanded indications improve our ability to provide the Celution® System to European hospitals in addition to the private-pay plastic and cosmetic surgery clinics,” said Marc H. Hedrick, M.D., president of Cytori. “These claims, coupled with our expanded focus on reimbursement, will make this technology more broadly available, not just for a wider range of procedures but to a greater population of patients.”
More than 370,000 women are diagnosed with breast cancer in Europe every year, the majority of whom are eligible to undergo a partial mastectomy, conserving as much of the unaffected breast as possible. Although the goal is to protect as much of the patient’s own tissue, the procedure can create a deformity that can be extremely difficult to repair, and most patients have limited reconstructive options. While there are several reconstructive options for women that have a full mastectomy, there is no uniformly accepted standard-of-care for partial mastectomy patients adopted by any country or medical society.
Interim results from Cytori’s RESTORE 2 trial show stem and regenerative cell-enriched fat grafting using the Celution®800 System resulted in a high sustained rate of physician (84% and 90%) and patient (73% and 70%) satisfaction and persistent improvements in overall outcomes of the procedure at six and 12 months respectively. The Celution® System is the only device approved under the European medical directive for separation and re-implantation of stem and regenerative cells residing in adipose tissue.
About Cytori
....
http://finance.yahoo.com/news/...evice-bw-850837773.html?x=0&.v=1
Der Wert der Meldung besteht ja wohl darin, dass die Hardware in der EU jetzt überhaupt erst verkauft werden darf! Da die Amis aber unter "Europa" vornehmlich "England" verstehen, wird die Kursbewegung sich in Grenzen halten. Leider hat all dies nichts mit der FDA zu tun...
Cytori's PureGraft System Receives European Approval for Fat Grafting Procedures
SAN DIEGO--(BUSINESS WIRE)--Cytori Therapeutics (NASDAQ: CYTX - News) has received European (CE Mark) approval for the PureGraft™ 250/PURE System for autologous fat grafting procedures, allowing Cytori to immediately commercialize the PureGraft™ product line to physicians in Europe. PureGraft™ will be sold as both a standalone product and as a complement to Cytori’s Celution® 800/CRS System in Europe.
The PureGraft™ System is an optimized tissue processing technology that is positioned at the forefront of the emerging fat grafting trend in the cosmetic and reconstructive surgery market. PureGraft™ replaces current non-standardized methods of graft preparation. Used independently, PureGraft™ rapidly and reliably produces optimal graft tissue for use in autologous fat grafting procedures. In combination with the Celution® 800/CRS, PureGraft™ lowers processing times and increases processing volumes, improving the utility and efficiency of Cytori’s core product for soft tissue applications.
“The addition of PureGraft™ to our product portfolio allows us to offer a broad spectrum of autologous fat grafting technologies in Europe. In addition to Celution® System, which enables cell-enriched fat grafting procedures, PureGraft™ fulfills the need for high quality, sterile tissue for fat grafting procedures, including body contouring,” said Eric Daniels, M.D., Cytori’s Managing Director in Europe and the Middle East.
Cytori will immediately begin commercializing PureGraft™ in Europe, through a combination of direct sales and distributors. In addition to the 27 countries of the EU, the CE Mark is followed by eight other countries and facilitates additional registrations around the world.
The PureGraft™ System sets a new standard for fat graft processing with its membrane-based tissue filtration combined with speed, simplicity, safety and precision. The PureGraft™ technology takes only 15 minutes to purify a fat graft ranging from 50 to 250 mL, removing excess and unwanted fluid, lipid, blood cells and debris in a controllable manner. The consumable-based system, used within the sterile field, “dialyzes” off everything but the purified fat tissue without centrifugation or other methods. The ease of use and simplicity of this innovative system sets it apart from other traditional fat grafting methods.
The PureGraft™ 250/PURE System received 510(k) marketing clearance for aesthetic body contouring from the U.S. FDA in January of this year. The product was formally launched at the meeting of the American Society of Aesthetic Plastic Surgeons in April 2010. In addition, Cytori received a Canadian medical device license for PureGraft™ in March 2010.
For more information, visit www.puregraft.com.
About Cytori ....
http://finance.yahoo.com/news/...stem-bw-2252723998.html?x=0&.v=1
Im Bereich um 5,40 könnte es wieder sehr zäh werden.
Wenn der Spetember über 4,40 hält, dann sehen wir auch bald über 6.
SAN DIEGO, CA -- (MARKET WIRE) -- 11/15/10 -- Adipose (fat) tissue-derived regenerative cells (ADRCs) obtained using the Celution® System demonstrated a statistically significant improvement in cardiac functional capacity (MVO2) at 18 months in Cytori's (NASDAQ: CYTX) PRECISE trial for chronic myocardial ischemia. New data from this trial were presented today at the American Heart Association Scientific Sessions 2010 in Chicago by Co-Principal Investigator Emerson C. Perin, MD, PhD, Director, Clinical Research for Cardiovascular Medicine, and Medical Director, Stem Cell Center, Texas Heart Institute.
The trial demonstrated the following outcomes in no-option chronic ischemic heart disease patients:
The statistically significant improvement in MVO2 (maximum oxygen consumption) in the cell treated group compared to the control group, first demonstrated at six months, is sustained at 18 months;
The statistically significant improvement in patients' ability to perform physical activity, as measured by metabolic equivalents (METS), in the cell treated group compared to the control group is sustained from 6 to 18 months; and
The procedure, which includes withdrawing fat tissue, separating out the regenerative cells using Cytori's Celution® System, and re-injecting the cells into the patient's heart, has previously been found to be safe and feasible, with no safety concerns emerging during the 18-month observation period.
ADRC treated patients had a lower cardiac mortality rate compared to the control. At an average follow up of 28 months, 2 of 6 placebo patients died of cardiac causes whereas 1 of 21 died in the cell treated group from cardiac causes. There were 2 patient deaths from non-cardiac causes in the cell treated group.
Based on this new information, Cytori will directly seek EU regulatory approval of this treatment for no-option chronic ischemia patients. The Company intends to file necessary submissions in early 2011.
Top Biotechnology Small Caps of the Week
Cytori Therapeutics, Inc. which belongs to the Biotechnology sector has a float of approximately 42.44M shares and approximately 50.51M shares outstanding. It
SAN DIEGO, CA and TOKYO -- (MARKET WIRE) -- 12/07/10 -- Cytori Therapeutics (NASDAQ: CYTX) and Astellas Pharma Inc. have entered into a strategic equity agreement to evaluate the potential of adipose derived stem and regenerative cells for the treatment of serious illnesses for which there is no fundamental treatment. Astellas will purchase approximately 1.43 million unregistered shares of Cytori common stock at $7.00 per share for net proceeds to Cytori of $10 million. As part of the agreement, Cytori granted Astellas the following additional rights:
Two year right of first refusal for a worldwide research, development and/or commercialization partnership using Cytori's products and technologies in the treatment of liver disease;
Non-voting observer seat on Cytori's board-of-directors; and
Participation in a newly formed scientific advisory board.
Per this agreement, Cytori and Astellas will further explore a collaboration for an advanced regenerative drug technology. The premium equity purchase will further support Cytori's ongoing clinical and commercial activities. The transaction is expected to close around December 13, 2010, subject to customary closing conditions.
Euro-Kurs:
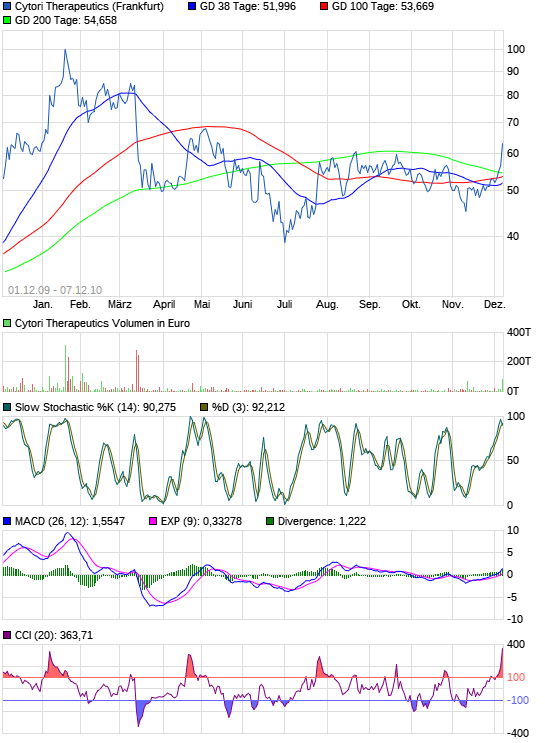
Was so oft wiederholt worden ist, muß doch einmal wahr werden!
Oder????
Geduld, Geduld, Geduld, auch Rom ist nicht an einem Tag erbaut worden.
ZUG, SWITZERLAND and SAN DIEGO, CA -- (MARKET WIRE) -- 03/02/11 -- Results from a European breast reconstruction trial using a new minimally invasive procedure to repair lumpectomy defects resulted in a sustained and substantial rate of physician and patient satisfaction at 12 months. The study, sponsored by Cytori Therapeutics (NASDAQ: CYTX), utilized the regenerative cells in the patient's own fat tissue, extracted at the time of the procedure using the Celution® 800/CRS System. The cells were then mixed back with a portion of the patient's own body fat, and this mixture of fat and cells was injected into the breast defect.
The trial, referred to as RESTORE-2, was a 71 patient prospective long-term breast reconstruction study. Specifically, 12 month physician satisfaction was 85% and patient satisfaction 75%, which is consistent with reported six month results. Physician and patient satisfaction criteria encompassed functional and cosmetic outcomes, namely breast deformity, breast symmetry, appearance of scarring, and skin pigmentation. With no generally accepted standard of care, there was no defined control for this trial. The comprehensive data are being prepared for peer-review and are expected to be publicly available later this year.
These important findings, along with the recent July 2010 European approval (CE Mark) on the Celution® System for breast reconstruction, are expected to increase availability to both hospitals and patients. Marketed in Europe as the RESTORE procedure, this novel approach is today funded at the local hospital level in an increasing number of European countries. This new data will further the Company's effort in securing specific national level payment in a number of key geographies. Patients and physicians in Europe can learn more about the RESTORE procedure at www.cellreconstruct.eu.
"We believe cancer treatment is incomplete without reconstruction," said Marc H. Hedrick, M.D., president of Cytori Therapeutics. "The RESTORE procedure has the potential to become the gold standard for lumpectomy defect repair, even in the context of radiation scarring, for which there is no accepted standard-of-care. The data from the study strengthens the long-term safety profile of this treatment and soundly shows efficacy in breast cancer patients."
Each year, approximately 450,000 European women are diagnosed with breast cancer. About 70% of these patients are eligible for breast conserving surgery, where only a portion of the breast is removed rather than the full breast. In these instances, patients are often left with a sizeable volume defect, scarring, and radiation damage. Because there is currently no widely accepted reconstruction procedure, offering a new option can address a major unmet medical need.
The Celution® System offers hospitals and clinics the only device that is approved in Europe for the extraction and re-implantation of regenerative cells from adipose tissue and it can do so in a cost-effective manner, without the need for cell-culture or time-consuming manual processing.
About the RESTORE Procedure
During the RESTORE procedure, fat is taken from the patient's stomach, hips, thighs, or other areas, by liposuction. Some of the tissue is used to extract the patient's own stem and regenerative cells which occur naturally inside the tissue, using Cytori's Celution® 800/CRS System. The extracted cells are then combined with some of the patient's own fat tissue, which forms a cell-enriched fat graft that is injected into the breast to restore its natural look and feel. In addition to providing an entirely natural option, the impact of post-operative scarring is greatly reduced due to the minimally invasive nature of this procedure.
About the RESTORE-2 Trial
RESTORE-2 is a post-marketing trial primarily intended to measure patient and physician satisfaction in reconstructing the breast utilizing the Celution® 800/CRS System. The trial took place at the following sites: Hospital General Universitario Gregorio Marañón in Madrid, Spain, Glasgow Royal Infirmary in Glasgow, Scotland, KU Leuven University Hospitals in Leuven, Belgium, Azienda Ospedaliero Universitaria Careggi in Florence, Italy, Instituto Valenciano Oncología in Valencia, Spain, Norfolk and Norwich University Hospital in Norwich, UK, and Jules Bordet Institute of Cancer in Brussels, Belgium.
BS Reporter / Chennai/ Hyderabad March 12, 2011, 0:44 IST
Apollo Hospitals is set to introduce a new stem cell-based treatment for the cosmetic and reconstructive industry in India, in six months.
The new therapy — regenerative cell-based therapy — is the resurgence of autologous fat grafting and the emergence of cell-enriched fat grafting technique, where a patient's own fat (adipose) is derived as stem and regenerative cells (ADRCs) to be used for the treatment.
“We have already tested this celution system-based cell-enriched fat grafting cosmetic procedures in Hyderabad and Delhi as part of the initial evaluation technology,” said Dr Sudhakar Prasad, Apollo Hospitals.
The celution system-based cell-enriched fat grafting cosmetic procedure is a patented technique of US-based Cytory Therapeutics. This would be used for treatment of neurological problems, heart attacks, facial scars, facial rejuvenation and wound healing.
Hari Prasad, chief executive, Apollo Hospitals, said, “We are following the Centre's guidelines. We went through trials and are awaiting the scientific committee's outcome.”
The technique is being used on 3,000 patients globally. Here, the outcome is much faster as the segregation process takes 90 minutes and the entire treatment takes around six months, he said.
The Indian cosmetic industry is a multi-million industry. “This initiative would bring another revolution in the cosmetic and healthcare industry,” Prasad said.
Cytory has invested $300 million in research and development so far.
http://www.business-standard.com/india/news/...metic-ind-soon/428187/
SAN DIEGO, CA--(Marketwire -12/19/11)- Cytori Therapeutics (NASDAQ: CYTX - News) filed its Investigational Device Exemption (IDE) application to begin a clinical trial of the Company's Celution® System for chronic myocardial ischemia (CMI).
ATHENA is a device-based safety and feasibility trial (Phase I/II) to investigate the use of autologous, clinical-grade adipose tissue-derived stem and regenerative cells (ADRCs), processed at the point-of-care with Cytori's proprietary Celution® System. The submission initiates a review process with the FDA before approval is granted to begin the trial.
Previously, Cytori reported six and 18 month trial data from PRECISE, a European clinical trial for this same indication showing statistically significant improvement in cardiac functional capacity, which was incorporated into the US IDE filing. In addition, Cytori has applied to expand its Celution® System CE Mark to include no-option CMI claims in Europe based on this data. Cytori is also enrolling a European pivotal trial using the Celution® System for acute heart attacks.
No-option CMI is estimated to affect approximately 6% of all coronary heart disease patients worldwide. In the U.S., more than one million patients are in this class with an annual cost of more than $10 billion. CMI patients typically have undergone multiple revascularization procedures, may suffer from serious abnormal heart rhythms, and have few therapeutic options. One goal of cell therapy in CMI is to revive hibernating heart muscle, which is living tissue that is not contributing to the pumping action of the heart.
About Cytori
Cytori is a leader in providing patients and physicians around the world with medical technologies that harness the potential of adult regenerative cells from adipose tissue. The Celution® System family of medical devices and instruments is being sold into the European and Asian cosmetic and reconstructive surgery markets but is not yet available in the United States. Our StemSource® product line is sold globally for cell banking and research applications. Our PureGraft® products are available in North America and Europe for fat grafting procedures. www.cytori.com
Cautionary Statement Regarding Forward-Looking Statements
This press release includes forward-looking statements regarding events, trends and business prospects, which may affect our future operating results and financial position, such as the successful initiation of an FDA review process to initiate a clinical trial of the Company's Celution® System for chronic myocardial ischemia, our efforts to expand our CE Mark, and our efforts to successfully revive hibernating heart muscle tissue. Such statements are subject to risks and uncertainties that could cause our actual results and financial position to differ materially. Some of these risks include clinical and regulatory uncertainties, such as those associated with the ATHENA clinical trial, including risks in the collection and results of clinical data, final clinical outcomes, dependence on third party performance, and other risks and uncertainties described under the "Risk Factors" in Cytori's Securities and Exchange Commission Filings. We assume no responsibility to update or revise any forward-looking statements to reflect events, trends or circumstances after the date they are made.
