Zwei Taipan-Empfehlungen: Ivanhoe, Genaera
Seite 1 von 1 Neuester Beitrag: 01.02.06 22:52 | ||||
| Eröffnet am: | 18.02.04 19:18 | von: Bleck | Anzahl Beiträge: | 7 |
| Neuester Beitrag: | 01.02.06 22:52 | von: Bleck | Leser gesamt: | 3.265 |
| Forum: | Hot-Stocks | Leser heute: | 3 | |
| Bewertet mit: | ||||
Ivanhoe (907373): Kanadischer Rohstoff-Wert (Öl, Gas), der in China gut positioniert ist. Kurs: 2,92 US$ Kurzfrist-Ziel: 7,50 US$ Langfrist-Ziel: 10 US$
Was meint ihr? Kennt ihr die Unternehmen näher?
Ivanhoe steht heute bei 2,21 USD, ein Minus von knapp 25%.
Bei Genaera sieht es anders aus. Kurs heute: 4,30 USD, ein knappes Plus von 5%. Zwischenzeitlich stand die Aktie allerdings deutlich höher (5 USD).
Es gab eine Kaufempfehlung für Genaera, die der Aktie 100% zutraut:
Die Analysten von WR Hambrecht Co bewerten in ihrer Analyse vom Freitag, 26. März 2004 die Aktie von Genaera Corp. nach wie vor mit dem Rating "Buy". Das Kursziel für die Aktie liegt momentan bei 9 $.
© finanzen.net
Hier Infos zu Ivanhoe
Ivanhoe Energy Inc. (Nasdaq.IVAN, Toronto.IE) is an independent energy company focused on creating shareholder value through finding and developing oil and gas reserves through the implementation of three major strategies: (1) conventional exploration and production of oil and gas, primarily in the U.S. and China (2) enhanced oil recovery and natural gas projects, and (3) monetization of stranded oil and gas reserves through the application of advanced technologies such as heavy-to-light oil upgrading and Gas-To-Liquids technologies. The company has established production in California, Texas and China.
© PR Newswire
Ivanhoe Energy Announces 2004 Drilling and Capital Program
BAKERSFIELD, CA, March 29 /PRNewswire-FirstCall/ -- David Martin, Chairman of Ivanhoe Energy, announced today that the company''s 2004 exploration and development plans provide for its participation in the drilling of a total of 65 wells in the USA and China. Ivanhoe''s share of the projected capital expenditure for the wells is estimated to be approximately US$52 million, compared to US$14.6 million in 2003. Three of the 65 wells already have been drilled and the company plans to participate in the drilling of 62 new wells over the balance of the year, of which 46 are development wells and 16 are exploration or appraisal wells.
In California, Ivanhoe plans to participate in development wells at the South Midway Sunset, Citrus and Sledge Hamar properties in the San Joaquin Basin and at the Knight''s Landing project in the Sacramento Basin. In Wyoming, Ivanhoe plans to begin drilling at the LAK Ranch project in the Powder River Basin in the second quarter of 2004. Internationally, Ivanhoe plans to participate in the drilling of 15 additional development wells at the Dagang field in the Bohai Basin in China.
The company''s exploration drilling is planned for the San Joaquin and Sacramento Basins in California, in East Texas and in China''s Sichuan Basin.
"The 2004 program adheres to our strategy of adding oil and gas reserves through the drilling of low-risk development wells and through selected exploration opportunities. We expect that the 2004 program will substantially increase our daily production of oil and gas by year end, generate recurring cash flow and add value to the company," said Mr. Martin. "The development program will be funded by the company''s recent equity financings, by funding agreements with third parties and by internally generated cash."
California ----------
South Midway Sunset - Ivanhoe has 51 producing wells in the South Midway Sunset project, with a working interest of 100%. Ivanhoe''s plan is to drill eight new wells in the second quarter of 2004 to increase production. Two of these wells will be exploration to test for new oil pools and six will be for ongoing development of existing pools. The wells will be steamed to increase production, which has proven successful in the existing operations.
Citrus (Lost Hills Field) - Ivanhoe has recently completed its first producing well, Citrus No. 1, with a working interest of 83%. Two additional wells are planned for the second quarter of 2004. The wells are expected to be drilled to a depth of approximately 8,700 feet and will test multiple producing zones that are productive in the offsetting area. If the initial three wells are successful, additional wells may be drilled on the 2,600-acre block.
Sledge Hamar (South Belridge Field) - Ivanhoe has one producing discovery well, Sledge Hamar 1-7, with a working interest of 40%. Six new wells are planned for 2004. The first of the six, the Sledge Hamar 2-7, will commence drilling in early April 2004 and test the Stevens interval that produces in the 1-7 well. Follow-up wells are planned to be drilled later this year to define the extent and productivity of additional oil zones that were encountered, but not tested, in the discovery well.
Knights Landing Area (Sacramento Basin) - Ivanhoe holds a 50% working interest in the 14,000-acre project and expects to have the initial pipeline connections to four new gas wells completed, operational and producing by late April 2004. In early May, the first 10-well drilling program is expected to commence with the drilling of three exploration wells and seven development wells. As a follow up to the initial drilling, the company may commence a second drilling campaign of up to an additional 10 wells in the second half of 2004.
North South Forty (San Joaquin Basin) - Ivanhoe holds a 50% working interest in three prospects that have been defined as a result of an extensive 3-D seismic program acquired in 2000 on lands west of the Belridge oil field. Three exploration wells are planned for the summer of 2004. Two of the wells are planned to be drilled to a depth of 1,500 feet and one is planned to be drilled to a depth of 3,500 feet.
Texas -----
East Texas - Two wells are planned under a farm-out agreement negotiated with Perryman Exploration Partners, which will operate the wells. Ivanhoe will have a carried working interest of 25% in the Malakoff and Catfish Creek prospects. The Malakoff well is planned to be drilled to a depth of 8,700 feet and the Catfish Creek well to a depth of 11,000 feet.
Wyoming -------
LAK Ranch - Ivanhoe has begun operations in the LAK Ranch joint development area and drilling is expected to start in the second quarter of this year. During the pilot phase, Ivanhoe will have an initial 30% working interest. The plan is to steam and produce an existing horizontal well and drill five additional steam injection wells to provide continuous steam to the reservoir, which should allow more oil to be produced from the horizontal well. Should the company decide to enter the next two phases of the contract, Ivanhoe''s working interest will increase to a maximum of 60%.
China -----
Dagang Project - Ivanhoe, through its wholly owned subsidiary Sunwing Energy Ltd., holds a 60% working interest in this oil development project. The development project commenced in late 2003, and the third new well is now being drilled. An additional 15 wells are planned to be drilled over the balance of this year. Over the next three years, the company expects to drill 115 new wells and work over 28 existing wells.
Zitong Project - Ivanhoe holds a 100% exploration working interest in a large contract area in the Sichuan gas basin. After interpretation of new seismic that is currently being acquired in the field, one exploration well is planned for late 2004.
Ivanhoe Energy trades on the NASDAQ SmallCap market with the ticker symbol IVAN and on the Toronto Stock Exchange with the symbol IE.
Information contacts: --------------------- In North America: Investors: Bill Trenaman +1-604-688-8323 Media: Bob Williamson +1-604-688-8323 In China: Patrick Chua +86-1370-121-2607 / +852-9193-4056 Website: http://www.ivanhoe-energy.com/
FORWARD-LOOKING STATEMENTS: This document includes forward-looking statements. Forward-looking statements include, but are not limited to, statements concerning estimates of expected drilling and development wells and associated costs, statements relating to anticipated capital expenditures, statements relating to increases in production, cash flows and values, statements relating to the continued advancement of Ivanhoe Energy''s projects and other statements which are not historical facts. When used in this document, the words such as "could," "plan," "estimate," "expect," "intend," "may," "potential," "should," and similar expressions are forward-looking statements. Although Ivanhoe Energy believes that its expectations reflected in these forward-looking statements are reasonable, such statements involve risks and uncertainties and no assurance can be given that actual results will be consistent with these forward-looking statements. Important factors that could cause actual results to differ from these forward-looking statements include the potential that the company''s projects will experience technological and mechanical problems, geological conditions in the reservoir may not result in commercial levels of oil and gas production, changes in product prices and other risks disclosed in Ivanhoe''s Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission.
Ivanhoe Energy Inc.
© PR Newswire
Genaera Receives Patent for Key Mucoregulator Therapeutic Target
PLYMOUTH MEETING, Pa., April 7 /PRNewswire-FirstCall/ -- Genaera Corporation today announced issuance by the United States Patent and Trademark Office of patent number 6,716,603, entitled "Nucleic Acids Encoding a Chloride Channel Protein" covering the composition of matter of the gene for the human calcium-activated chloride channel (hCLCA1) and methods for producing the encoded protein which is believed to be important as a mucoregulator target in a variety of important medical conditions. This patent also covers vectors and transformed cells containing the hCLCA1 gene product and their use for recombinant production of the hCLCA1 protein.
The hCLCA1 chloride channel is a therapeutic target and component of Genaera''s second genomics-based development program that is believed to regulate abnormal mucus production and epithelial inflammation. This therapeutic target is potentially associated with the pathogenesis of a variety of disorders including cystic fibrosis, chronic bronchitis, asthma, and other chronic and acute respiratory, sinus and gastrointestinal disorders.
"This patent is a critical component for Genaera in solidifying our intellectual property strategy surrounding our mucoregulator program," noted Roy C. Levitt, MD, President and Chief Executive Officer. "We are pleased to secure this important patent as we believe hCLCA1 is a critical therapeutic target for many conditions associated with the overproduction of mucus. We are leaders in this field and will continue to inform companies working in this area of our substantial patent position, as well as discuss the potential for a strategic alliance."
The mucoregulator program is Genaera''s second product development program based on its genomics discoveries. Based on the role of the hCLCA1 chloride channel in respiratory diseases, such as cystic fibrosis, the Company has developed LOMUCINO. LOMUCINO (talniflumate) is an oral treatment intended to block the hCLCA1-dependent mucus overproduction present in respiratory, sinus and gastrointestinal disorders, and thereby provide a new strategy for treating disorders associated with abnormal mucus production. LOMUCIN(TM) Phase II clinical development is supported in part by a grant of up to $1.7 million from Cystic Fibrosis Foundation Therapeutics Inc.
There is an extensive unmet medical need for a therapy that can prevent abnormal mucus production in a variety of disorders. Chronic sinusitis is one of the most common reasons for physician visits in the United States, with 35 million cases per year. It is thought that many of the symptoms of chronic sinusitis result from excess mucus production. According to the National Institute of Allergies and Infectious Disease and the American Lung Association, there are more than 65 million patients suffering from diseases where mucus overproduction may be hCLCA1 mediated, including 17 million asthmatics and 35 million respiratory allergy sufferers. Mucus overproduction and small airway plugging is one of the lethal sequelae of asthma and excess mucus production is also associated with COPD and chronic bronchitis. Cystic fibrosis is characterized by a dramatic increase in mucus production, and mucoregulator therapy may be beneficial in this condition as well.
Genaera Corporation is a biopharmaceutical company committed to developing medicines for serious diseases from genomics and natural products. Research and development efforts are focused on anti-angiogenesis and respiratory diseases. Genaera has three products in development addressing substantial unmet medical needs in major pharmaceutical markets. These include squalamine, an anti-angiogenesis treatment for cancer and eye disease; interleukin-9 antibody, a respiratory treatment based on the discovery of a genetic cause of asthma; and LOMUCINO, a mucoregulator to treat the overproduction of mucus and secretions involved in many forms of chronic respiratory disease. For more information on Genaera, visit the company''s website at http://www.genaera.com/.
This announcement contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties, known and unknown. Forward-looking statements reflect management''s current views and are based on certain expectations and assumptions. You may identify some of these forward-looking statements by the use of words in the statements such as "anticipate," "develop," "expect," "believe", and "continue," or other words of similar meaning. Genaera''s actual results and performance could differ materially from those currently anticipated and expressed in these and other forward-looking statements as a result of a number of risk factors, including, but not limited to, the risk that Genaera is unable to close the financing referred to above; Genaera''s history of operating losses since inception and its need for additional funds to operate its business; the costs, delays and uncertainties inherent in scientific research, drug development, clinical trials and the regulatory approval process; the risk that clinical trials for Genaera''s product candidates, including LOMUCIN, may not be successful; the risk that Genaera may not obtain regulatory approval for its products, whether due to adequacy of the development program, the conduct of the clinical trials, changing regulatory requirements, different methods of evaluating and interpreting data, regulatory interpretations of clinical risk and benefit, or otherwise; Genaera''s reliance on its collaborators, in connection with the development and commercialization of Genaera''s product candidates; market acceptance of Genaera''s products, if regulatory approval is achieved; competition; general financial, economic, regulatory and political conditions affecting the biotechnology and pharmaceutical industry; and the other risks and uncertainties discussed in this announcement and in Genaera''s filings with the U.S. Securities and Exchange Commission, all of which are available from the Commission in its EDGAR database at http://www.sec.gov/ as well as other sources. You are encouraged to read these reports. Given the uncertainties affecting development stage pharmaceutical companies, you are cautioned not to place undue reliance on any such forward-looking statements, any of which may turn out to be wrong due to inaccurate assumptions, unknown risks, uncertainties or other factors. Genaera does not intend (and it is not obligated) to publicly update, revise or correct these forward-looking statements or the risk factors that may relate thereto.
Genaera Corporation
© PR Newswire
Ivanhoe Energy stock up on production forecast
Last Updated Wed, 21 Apr 2004 16:36:16
BAKERSFIELD, CALIF. - Shares of Ivanhoe Energy Inc. (TSX:IE) moved up sharply Wednesday after the company said production is "expected" to at least triple and could increase more than fourfold this year.
Before the market opened, Ivanhoe said estimated production this year will range between 3,400 and 4,400 barrels of oil equivalent a day (BOE/D), compared with actual production of 1,035 BOE/D at the end of 2003.
The stock was up 84 cents or more than 26 per cent to $4.02. The 52-week range is 53 cents to $10.40.
The company is planing to drill up to 65 wells this year, David Martin, chairman, said in a statement.
"The 2004 drilling program is part of a multi-year effort to grow the company through development of its existing resource base," he said.
The company's focus is China and California.
As of last May, Ivanhoe was controlled by famous mining promoter Robert Friedland, who owned 32 per cent of the stock, and other officers and directors, who held 36 per cent, a regulatory filing shows.
Friedland is best known for finding the Voisey's Bay nickel deposit in Newfoundland, now being developed by Inco.
www.cbc.ca
Written by CBC News Online staff
PLYMOUTH MEETING, Pa., April 22 /PRNewswire-FirstCall/ -- Genaera Corporation today announced that the U.S. Food and Drug Administration (FDA) has cleared Genaera''s investigational new drug (IND) application for its systemically administered anti-angiogenic drug, squalamine. The IND covers treatment of neovascular diseases of the eye, including subfoveal choroidal neovascularization associated with age-related macular degeneration, known as "wet" AMD. Genaera also announced that they plan to begin Phase II trials in AMD this quarter, and these Phase II trials will run concurrently with the start of Phase III trials in AMD, beginning in early 2005.
"One of Genaera''s highest priorities is expediting squalamine''s commercialization timeline for wet AMD. We are extremely pleased with the FDA''s feedback allowing us to proceed into large-scale clinical trials, including Phase III," commented Roy C. Levitt, MD, President and Chief Executive Officer. "Squalamine has tremendous potential to be a safe, effective, and less invasive anti-angiogenic therapy for this devastating eye disease. At four months after therapy with squalamine, 100% of patients showed stable or improved vision. Our initial Phase I/II clinical trial data including safety and efficacy are comparable or superior to any published or presented results at a similar stage of development for other wet AMD anti-angiogenic therapies, such as Macugen(TM) and Lucentis(TM)."
Dr. Levitt further commented, "Our plans are to focus on first-line therapy for AMD. Genaera expects to complete three Phase II clinical trials for squalamine in AMD. These Phase II trials will be running while we are actively preparing for our anticipated Phase III trials."
The cornerstone of Genaera''s Phase II studies is an exploratory trial designed to evaluate the safety and efficacy of squalamine in 100 patients with AMD over a two year period. This Phase II multi-center, randomized, double masked, controlled study will evaluate two dose levels of squalamine (20 mg or 40 mg) given once weekly for four weeks, followed by maintenance doses once every four weeks until week 48. At the end of 48 weeks of therapy, each patient will be followed for 12 months. Analyses from this study are expected to be used in coordinating Phase III activities.
A second exploratory Phase II trial is designed to evaluate the effects of two different doses of squalamine in combination with an initial Visudyne(R) treatment in 30 patients with AMD. Specifically, this study will evaluate the potential benefits of squalamine pretreatment on the actions of Visudyne(R) and the added potential benefit of dosing squalamine after Visudyne(R) to inhibit the potentially detrimental effects of the VEGF ''burst'' that commonly occurs after Visudyne(R) treatment. The multi-center, randomized, controlled, masked study includes monthly squalamine maintenance therapy, and twelve months subsequent follow-up for each patient.
Genaera will also conduct a Phase II pharmacokinetic and safety trial that will evaluate 18 patients with AMD at three different doses of squalamine over four months. In this open-label, parallel group study, squalamine will be administered intravenously at three doses, once weekly for four weeks.
Conference Call
At 11:00 a.m. ET today, Genaera will webcast a conference call hosted by Roy C. Levitt, MD, President and Chief Executive Officer, to discuss Genaera''s clinical development plans for squalamine in AMD.
Those who wish to participate in the conference call may telephone (877) 407-8031 approximately 10 minutes before the start time. A slide presentation will be available on the Internet via Vcall. To access the live or archived call via the Internet, please log onto: http://www.genaera.com/ or http://www.vcall.com/CEPage.asp?ID=87897. Please connect to the site prior to the conference call to ensure adequate time for any software download that may be needed to hear the webcast.
Squalamine Mechanism of Action
Squalamine directly interrupts and reverses multiple facets of the angiogenic process. Working within activated endothelial cells, squalamine inhibits growth factor signaling including VEGF, integrin expression, and reverses cytoskeletal formation, thereby resulting in endothelial cell inactivation and apoptosis. Systemically administered squalamine inhibits abnormal angiogenesis in rodent models of retinopathy of prematurity, and the development of choroidal neovascular membranes in rat models of AMD. Additional preclinical studies have demonstrated that systemic squalamine administration is effective in reaching abnormal ocular blood vessels in primates, and leads to partial regression and inhibition of new abnormal vessels in the eye. These results support that squalamine may have a role in the treatment of human choroidal neovascular membrane formation that underlies the pathology of wet AMD.
About AMD
Angiogenesis resulting from AMD is the leading cause of legal blindness among adults age 50 or older in the Western world. About 25-30 million people are affected globally. This number is expected to triple over the next 25 years.
AMD occurs in two types: the "dry" form and the more severe "wet" form. Wet AMD is caused by the growth of abnormal blood vessels, or choroidal neovascularization, under the central part of the retina, the macula. Dry AMD, or the avascular form is the more common and milder form of AMD, accounting for 85% to 90% of all cases. Dry AMD results in varying forms of sight loss and may or may not eventually develop into the wet form. Although the wet form of AMD accounts for only 10% to 15% of all AMD, the chance for severe sight loss is much greater. It is responsible for 90% of severe vision loss associated with AMD. Approximately 500,000 new cases of wet AMD are diagnosed annually worldwide. In North America alone, approximately 200,000 new cases of wet AMD are diagnosed each year. Wet AMD is caused by the growth of abnormal blood vessels, or choroidal neovascularization, under the central part of the retina, the macula.
Genaera Corporation is a biopharmaceutical company committed to developing medicines for serious diseases from genomics and natural products. Research and development efforts are focused on anti-angiogenesis and respiratory diseases. Genaera has three products in development addressing substantial unmet medical needs in major pharmaceutical markets. These include squalamine, an anti-angiogenesis treatment for cancer and eye disease; interleukin-9 antibody, a respiratory treatment based on the discovery of a genetic cause of asthma; and LOMUCINO, a mucoregulator to treat the overproduction of mucus and secretions involved in many forms of chronic respiratory disease. For more information on Genaera, visit the company''s website at http://www.genaera.com/.
This announcement contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties, known and unknown. Forward-looking statements reflect management''s current views and are based on certain expectations and assumptions. You may identify some of these forward-looking statements by the use of words in the statements such as "anticipate," "believe," "continue," "develop," "expect," "plan" and "potential" or other words of similar meaning. Genaera''s actual results and performance could differ materially from those currently anticipated and expressed in these and other forward-looking statements as a result of a number of risk factors, including, but not limited to; Genaera''s history of operating losses since inception and its need for additional funds to operate its business; the costs, delays and uncertainties inherent in scientific research, drug development, clinical trials and the regulatory approval process; the risk that clinical trials for Genaera''s product candidates, including, squalamine, may not be successful; the risk that Genaera may not obtain regulatory approval for its products, whether due to adequacy of the development program, the conduct of the clinical trials, changing regulatory requirements, different methods of evaluating and interpreting data, regulatory interpretations of clinical risk and benefit, or otherwise; Genaera''s reliance on its collaborators, in connection with the development and commercialization of Genaera''s product candidates; market acceptance of Genaera''s products, if regulatory approval is achieved; competition; general financial, economic, regulatory and political conditions affecting the biotechnology and pharmaceutical industry; and the other risks and uncertainties discussed in this announcement and in Genaera''s filings with the U.S. Securities and Exchange Commission, all of which are available from the Commission in its EDGAR database at http://www.sec.gov/ as well as other sources. You are encouraged to read these reports. Given the uncertainties affecting development stage pharmaceutical companies, you are cautioned not to place undue reliance on any such forward-looking statements, any of which may turn out to be wrong due to inaccurate assumptions, unknown risks, uncertainties or other factors. Genaera does not intend (and it is not obligated) to publicly update, revise or correct these forward-looking statements or the risk factors that may relate thereto.
Genaera Corporation
© PR Newswire
(©GodmodeTrader - http://www.godmode-trader.de/)
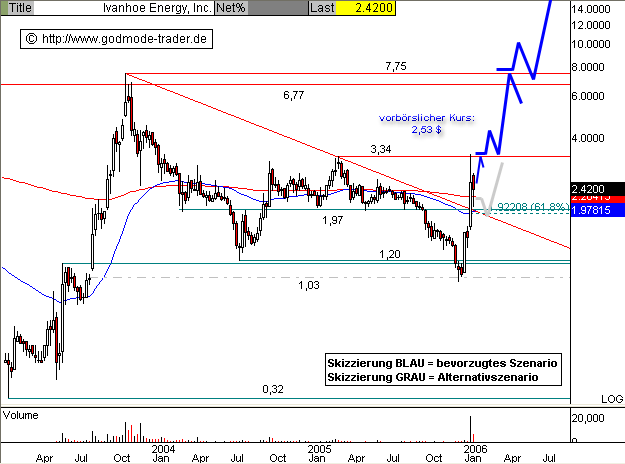
Ivanhoe Energy (IVAN / ISIN: CA4657901035) : 2,53 $ (+4,55%)
Aktueller Wochenchart (log) seit Januar 2003 (1 Kerze = 1 Woche).
Diagnose/Prognose: Die IVANHOE Aktie konnte im Sommer 2005 keine Aufwärtsdynamik entwickeln und begab sich mit dem Rutsch unter die Horizontalunterstützung bei 1,97 $ auf den Weg des Alternativszenarios. Im Dezember erreichte die Aktie den horizontalen Unterstützungsbereich bei 1,20 $ und unterschritt diesen Bereich kurzzeitig, um das noch offene Gap bei 1,03 - 1,10 $ zu schließen. Im Januar gelang dann die Rückkehr über 1,20 $ und anschließend sogar über 1,47 $. Damit notiert die Aktie nun wieder auf der Bullenweide und springt heute vorbörslich nochmals um über 3% nach oben. Die Eröffnung wird im Bereich von 2,53 $ liegen. Nach der letztwöchigen Korrektur kann die Aufwärtsbewegung prinzipiell wieder aufgenommen werden. Ein kurzfristiges Kaufsignal ergibt sich beim Überwinden des Horizontalwiderstandes bei 3,34 $. Erstes Ziel wäre dann der Widerstandsbereich am AllTimeHigh bei 6,77 - 7,55 $ als langfristige BUY Trigger Marke. Alternativ korrigiert die Aktie nochmals bis an den zentralen Unterstützungsbereich aus Horizontalunterstützung, 61,8% Fibonacci Retracement der Aufwärtsbewegung seit Anfang Januar sowie EMA200 auf Tages- und EMA50 auf Wochenbasis bei 1,92 - 1,98 $. Erst ein Rückfall darunter trübt das mittelfristig bullische Szenario ein.
www.godmodetrader.de