Introgen - ein zukünftiger Highflyer?
Seite 1 von 1 Neuester Beitrag: 19.04.04 20:40 | ||||
| Eröffnet am: | 11.12.03 01:04 | von: ipollit | Anzahl Beiträge: | 11 |
| Neuester Beitrag: | 19.04.04 20:40 | von: Quietschente | Leser gesamt: | 4.445 |
| Forum: | Börse | Leser heute: | 1 | |
| Bewertet mit: | ||||
INGN ist bei einer MK von etwa 200 Mio. USD im Nasdaq Biotech Index vertreten. 10 Jahre alt und seit 3 Jahren an der Börse sind INGN's Umsätze minimal und es fallen noch moderate Verluste an, was bei Biotechs ohne Produkte am Markt den Normalfall ist:
"During the quarter ended September 30, 2003, cash and cash equivalents decreased $4.0 million. At September 30, 2003, Introgen had cash and cash equivalents of $22.6 million.
For the nine months ended September 30, 2003, Introgen reported a net loss of $13.9 million, or $0.62 per share, compared to a net loss for the nine months ended September 30, 2002 of $20.5 million, or $0.96 per share. Revenue for the nine months ended September 30, 2003 was $302,000, compared to revenue for the nine months ended September 30, 2002 of $1.0 million. Operating expenses were $15.5 million for the nine months ended September 30, 2003 compared to $22.3 million for the nine months ended September 30, 2002." (Letzte Woche (3.12.) haben sie erfolgreich eine Kapitalerhöhung von 20 Mio. USD (Kurs 7 USD) durchgeführt, so dass die Cash-Situation sich wieder gebessert hat...)
Doch dieser Zustand könnte sich durch ihren aktuell wichtigsten Kandidaten in der Pipeline bald ändern:
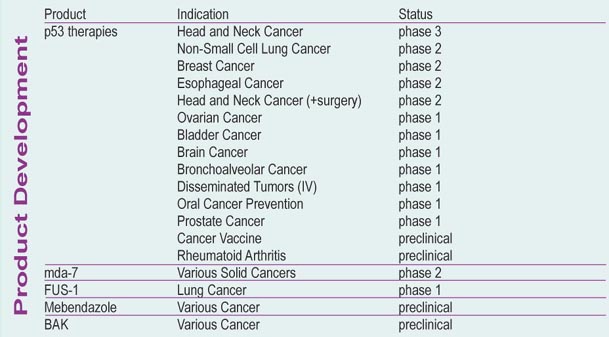
Advexin befindet sich für die Indikation Krebs im Kopf- und Halsbereich bereits in fortgeschrittener Phase III. Im nächsten Jahr wird bei positiven Ergebnissen bei der FDA die Zulassung beantragt, so dass, wenn alles gut geht, Anfang 2005 die ersten Umsätze generiert werden können (Advexin hat von FDA fast-track-Status). Allerdings wird die Sache nicht leicht, da INGN mit ihrer Technologie der Gentherapie (Vektoren um therapeutische Gene in die Zelle zu transportieren) absolutes Neuland betritt. Bei Erfolg von Advexin hat aber INGN meiner Meinung nach noch großes Potential... Advexin läßt sich gegen viele weitere Tumore einsetzen und wird daneben als Krebs-Vakzine (INGN 225) entwickelt... ein weiterer Kandidat INGN 241 (Genfähre für mda-7) basiert auf dem gleichen Prinzip (Phase II). In Phase I und Präklinik befinden sich noch weitere Kandidaten.
Ist Advexin erfolgreich, so hat es Blockbusterpotential... wenn das Prinzip der Gentherapie fehlschlägt verliert INGN seine ganze Pipeline - d.h. neben der großen Chance gibt es hier auch ein beträchtliches Risiko!
Hat sich schonmal jemand mit Introgen beschäftigt? Infos oder Meinungen?
mfg ipollit :-)
By Bill Mann
December 8, 2003
Denis Leary may turn out to be disappointed. His 1992 comedy album, "No Cure for Cancer," may be proven to be wrong by modern science. There are dozens of pharmaceutical and biotechnology companies that are actively seeking cures for cancer -- each has its own specialty. One of the coolest treatments that I have come across is entering Phase II trials. It's called Advexin, and the company that is developing it is Introgen Therapeutics (Nasdaq: INGN).
Advexin's already gone into Phase II trials (during which the therapy is tested on patients with the disease) for breast, esophagus, and lung cancers, and Phase III for head and neck cancers. Each of these has a relatively high rate of mortality. The latest Advexin trial is for mouth and throat cancers, and it delivers the drug using, of all things, a mouthwash.
One of the problems with many cancer therapies is that the treatment is sometimes nearly as bad as the disease. Had you suggested to me years ago that cancer treatments would be well advanced by this time, I would have readily believed you. But the thought that some deadly forms of cancer might be treated topically, and not invasively, is mind-blowing.
Every cell in the human body has a protein called p53, also known as the "cellular policeman," which detects whether something is amiss in a cell. If so, p53 causes the cell to destroy itself. With many forms of cancer, this process is suppressed, so cells that ought to kill themselves fail to do so. Tumors form when these mutated cells multiply instead. Medicine has long understood the role of rapid reproduction of cells in cancer, but the role of the failure of cells to police themselves has only recently become more understood.
Finding a way to reintroduce p53 to mutated cells would allow them to do what nature intended them to do in the first place -- put themselves to sleep. This could be substantially less toxic than existing therapies that destroy both cancerous and healthy cells indiscriminately.
Introgen's rights include an adenoviral delivery system for p53. Just in the way that viruses introduce influenza, malaria, or other diseases to a system, some can also be altered to introduce p53.
Introgen's financial statements are awful. Like most biotechnology companies, it has minimal revenues prior to approval of its drugs, though it does have plenty of cash and minimal debt. But the delivery system is so ingenious that I was intrigued enough to ask a Georgetown University oncologist, Dr. Jimmy Hwang, what he knew about p53, Advexin, and Introgen.
Dr. Hwang explained that he found it interesting that there is substantially more information about this therapy in business publications than in medical journals, and noted that a drug's passing to Phase II only means "that the drug is safe." Still, he called the reintroduction of p53 to be a sort of Holy Grail for cancer treatment. From an investment standpoint, Introgen most likely falls under the low confidence/high potential return category.
Still, the thought of a swish that can kill cancer cells is something I find completely fascinating.
*****************************************
Introgen prepares for boom
Biotech company moving toward full production of its cancer drug
By Colin Pope
Austin Business Journal
Dec. 8 — After laying relatively dormant for a decade, Introgen Therapeutics Inc. is gearing up for a growth spurt as its novel cancer-fighting treatment is on the verge of being approved by federal regulators.
At Introgen's manufacturing plant in Houston, workers already are stockpiling the promising Advexin drug for commercial sale -- possibly as early as late 2004.
At the corporate headquarters in Austin, dealmakers are reaching out to pharmaceutical giants and other companies that could market Advexin worldwide. They're also considering ways to establish an internal sales force. The company's regulatory experts are preparing to ask the U.S. Food and Drug Administration for permission to sell Advexin, and Introgen's financial team has strengthened the company's balance sheet by offering $20 million more of common stock.
"There's no question," founder and CEO David Nance says, "we're getting ready for a very important year for Introgen."
Introgen [Nasdaq: INGN] has waited a decade for something many Austin startups demand within a matter of months -- a product launch.
After 10 years, $500 million and a plethora of promising results from FDA studies, Introgen's flagship product -- a drug called Advexin -- appears to be on the brink of distribution.
Advexin's primary target now, head and neck cancer, has a peak sales opportunity as high as $500 million, according to the New York-based investment bank Rodman & Renshaw Inc. But Navdeep Jaikaria, the Rodman & Renshaw analyst who follows Introgen, says the overall market for Introgen's gene-based drug easily could top $1 billion.
That's because oncologists have a tendency to use newly approved cancer drugs to treat other forms of the disease. So although Advexin might have the FDA's approval only for head and neck cancer next year, the drug could be used to treat other types of cancer.
The medical community already is keeping close tabs on Introgen because of Advexin's potential effect on a variety of cancers such as lung, breast, prostate, ovarian, bladder and brain cancers, says Phil Nadeau, an analyst at SG Cowen Securities Corp. in New York.
Unlike chemotherapy, radiation and surgery, Introgen's cancer combatant involves the p53 gene, which humans naturally produce. The p53 gene recognizes that cells aren't normal and either fixes the damage or kills the cell when it is injected into a tumor. It leaves the healthy cells alone.
Introgen has just a few more steps to get Advexin on the market. The most signific ant move is expected sometime next year, Nance says, when Introgen submits to the FDA a biologics license application.
After the application is turned in, federal regulators have about six months to tell Introgen it can or can't market Advexin. Once Introgen gets the OK, Nance says, the stockpile of Advexin in Houston will be shipped out almost immediately.
"We'll begin the distribution right away," Nance says. "Every day it's not on the market, we're missing an opportunity -- not just for sales, but to save patients."
Introgen already is talking to potential marketing partners that could sell Advexin in Europe and Asia, Nance says. Potential partners include nearly all the major pharmaceutical firms and a handful of large biotech companies that have marketing arms, such as Genentech Inc. or Amgen Inc., both of which are based in California but have worldwide operations.
Although a partner will sell Advexin abroad, Introgen is looking at creating an internal sales team to market Advexin to cancer centers and oncologists in the United States and Canada, Nance says. Those jobs might be spread between Austin and Houston, he says.
Introgen now has about 80 employees -- only the executive team works in Austin -- but Nance expects to add to the workforce here and in Houston next year.
After selling $20 million worth of shares late last month, Nance says Introgen has about $40 million of cash on hand, which is enough to cover operating costs until about mid-2004. Introgen already has regulators' permission to offer $80 million more of stock when the need arises.
So far, as much as $500 million has been invested to bring Advexin to the market, which begs the question: How could Introgen afford to spend half a billion dollars over a 10-year period?
It couldn't. Introgen has only doled out about $150 million for research and development. The other $350 million came from other cancer antagonists, such as the National Cancer Institute, the Southwest Oncology Group in San Antonio and even French drug giant Aventis.
Meanwhile, Introgen was able to hang onto 100 percent of Advexin's commercial rights, Nance says.
"Introgen really leveraged the investments of others," Nance adds. "We were fortunate to not have to use all of our cash on development."
Once revenue from Advexin starts rolling in, Introgen will tackle its biggest project yet: a new and larger manufacturing plant that can produce Advexin plus Introgen's other treatments already in the FDA pipeline, Nance says.
"The question is then: Where do we want to site a larger production facility?" Nance says.
The answer: He doesn't know yet.
But one thing is for sure, Nance says. The multimillion-dollar plant, which will at first measure about 100,000 square feet, will need to have plenty of room to grow.
"I sure would like to keep it in Texas, but I haven't gone beyond that," Nance says. "In '05 or '06, we'll start to give it serious consideration."
**************************
Austin's Introgen gets cancer drug on FDA's fast track
Austin company could be first to sell gene-based drug
By Robert Elder Jr.
AMERICAN-STATESMAN STAFF
Thursday, September 18, 2003
Federal regulators put the leading cancer drug from Introgen Therapeutics Inc. on the fast track for approval Wednesday, bringing the Austin company closer to tapping into an estimated $1 billion market for its gene-based drugs.
The U.S. Food and Drug Administration granted fast-track status for Advexin, an Introgen drug used to treat head and neck cancer.
Most importantly, the news offers hope for people with cancer. It also brightens the prospects and public profile of Introgen, perhaps the most prominent of Austin's handful of biotechnology concerns. Until a recent sale of additional stock to investors, Introgen was running low on cash while working feverishly on advanced trials of Advexin. The FDA news sent Introgen's stock surging 23 percent to close at $10.66 per share, a 52-week high.
The FDA's decision doesn't necessarily mean it's more likely to approve Advexin, but fast-track status shortens the approval process by letting a company file its application piecemeal. Typically, fast-track drugs come up for approval within six months, compared with about 10 months for the normal process.
Introgen could be selling Advexin for head and neck cancer by early 2005. If that happens, Introgen is likely to be the first company in the world to sell a gene-based drug.
The FDA grants fast-track status for drugs that show they have the potential to address life-threatening diseases for which no treatments are available. There is no approved drug treatment for head and neck cancer, which affects about 40,000 Americans per year.
With other uses for Advexin being tested -- including promising results for lung and ovarian cancers -- the overall market for Introgen's line of gene-based drugs could hit $1 billion, said Navdeep Jaikaria, senior biotechnology analyst with Rodman & Renshaw in New York.
David Nance, Introgen's chief executive and president, wouldn't comment on that figure, but he said Advexin "should experience significant growth" once approved because it can be used in conjunction with surgery and other methods of treating cancers.
"It has a bright future," he said.
If Advexin meets those lofty sales projections, it would give Austin a big-name public biotechnology company and boost the region's negligible profile as a biosciences center, although Introgen has just a handful of its 50 employees in Austin, mostly corporate executives.
Its research facilities are in Houston, and the company's roots date to research done at the University of Texas M.D. Anderson Cancer Center.
Nance, who was a founding investor in the company, said Advexin's potential success in the market at large will "validate the idea that a company headquartered in Austin can develop drugs in the absence of a large university hospital" and other major health-care research facilities.
Jaikaria estimated Advexin drug sales would reach $215 million by 2007. He said fast-track status essentially "lowers the bar for regulatory approval."
"Biotech investors understand that," he said, referring to the spike in Introgen's share price Wednesday.
Advexin is the most advanced experimental drug from Introgen, a 10-year-old company that went public in October 2000.
Advexin delivers a gene into the body that halts the growth of cancer cells while sparing healthy ones. The drug delivers a protein called p53, which is thought to kill cells containing DNA damage -- a symptom of cancer.
Although the FDA decision concerns the use of Advexin for head and neck cancers, oncologists often use newly approved cancer drugs to treat other forms of the disease. That means sales of new drugs can explode once they hit the market.
One recent example is Idec Pharmaceutical Corp.'s best-selling drug Rituxan, which was approved to treat a relapse of a specific type of non-Hodgkin's lymphoma. It rocketed to more than $1 billion in sales a few years after its 1997 introduction because of other uses.
mfg ipollit ;-)
Greetz
Abstract #42, "Combination therapy of Ad-mda7 and Herceptin increases apoptosis in Her-2/neu-overexpressing breast cancer" described preclinical studies of a promising combination therapy for breast cancer. INGN 241 and Herceptin(R) (trastuzumab) were evaluated as single agents and in combination. The data show that INGN 241 and Herceptin each reduced viability and tumor growth of HER2-positive cancer cell lines when used as monotherapy. INGN 241, but not Herceptin, demonstrated a similar growth inhibitory effect in HER2- negative cells. In HER2-positive cells, INGN 241 significantly enhanced the anti-tumor activity of Herceptin, resulting in increased growth inhibition. This appears to be the result of enhanced down-regulation, in the combination regimen, of specific proteins associated with a poor prognosis in patients with breast cancer. The complete data from this study are expected to be published in a peer-reviewed scientific publication later this year.
Two additional abstracts, "Adenoviral MDA-7 induces apoptosis in p53 resistant lung cancer cells through PKR induction" (Abstract #88) and "Histone deacetylase inhibition enhances Ad-mda-7 transgene expression and apoptosis induction in lung cancer cell lines" (Abstract #91) discussed studies evaluating two molecular pathways involved in INGN 241-induced cancer cell death. Data from Abstract #88 indicate that INGN 241 induces apoptosis (programmed cell death) through the activation of the PKR pathway, resulting in increased expression of key mediators of apoptosis. This study suggests that INGN 241 can overcome resistance to anti-cancer drugs by exploiting novel cancer-killing mechanisms. Data from Abstract #91 identify a novel approach to increasing anti-tumor activity in cancer cells treated with INGN 241. In these studies, the addition of an inhibitor of histone deacetylase four hours after treatment with INGN 241 resulted in increased amounts of MDA-7 protein and increased apoptosis in three different lung cancer cell lines. Histone deacetylase inhibitors include small chain fatty acids such as sodium butyrate and have shown promise as anti-cancer drug candidates. Complete data from the studies reported in Abstract #88 also are expected to be published later this year.
Sunil Chada, Ph.D., Introgen''s director of research and development, said, "A variety of preclinical studies of INGN 241 are providing important data that may enhance the clinical efficacy of this promising cancer therapy candidate. The addition of small molecules to INGN 241 regimens increases the rate of tumor cell killing and may allow us to achieve clinically meaningful effects at lower doses of INGN 241. Additionally, the promising results from the combination of INGN 241 and Herceptin highlight the broad utility of INGN 241 as a potential component of a variety of cancer regimens. We believe that these data will help to strengthen our clinical development program for INGN 241 and look forward to sharing the complete data set from these studies with our colleagues in the oncology community when they are published later this year."
The mda-7 gene was discovered by the laboratory of Dr. Paul B. Fisher, professor of clinical pathology and the Michael and Stella Chernow Urological Cancer Research Scientist in the Departments of Neurological Surgery, Pathology and Urology at Columbia University. Introgen holds an exclusive worldwide license to the mda-7 gene from the Corixa Corporation.
Introgen is a leader in the development and production of gene-based drugs for the treatment of cancer and other diseases. Introgen maintains integrated research, development, manufacturing, clinical and regulatory departments and operates a commercial-scale, CGMP manufacturing facility.
Certain statements in this press release that are not strictly historical may be "forward-looking" statements, which involve risks and uncertainties. Such forward-looking statements include, but are not limited to, those relating to Introgen''s future success with its clinical development program with INGN 241 for the treatment of cancer. There can be no assurance that Introgen will be able to commercially develop gene-based drugs, that necessary regulatory approvals will be obtained or that any clinical trials or studies undertaken will be successful or that the proposed treatments will prove to be safe and/or effective. The actual results may differ from those described in this press release due to risks and uncertainties that exist in Introgen''s operations and business environment, including, but without limitation, Introgen''s stage of product development and the limited experience in the development of gene-based drugs in general, Introgen''s dependence upon proprietary technology and current competition, history of operating losses and accumulated deficits, reliance on collaborative relationships, and uncertainties related to clinical trials, the safety and efficacy of Introgen''s product candidates, the ability to obtain the appropriate regulatory approvals, patent protection and market acceptance, as well as other risks detailed from time to time in Introgen''s filings with the Securities and Exchange Commission, including its annual report on Form 10-K filed on March 31, 2003 and its quarterly report on Form 10-Q filed on November 14, 2003, and its prospectus supplement filed with the SEC pursuant to Rule 424(b)(2) on November 26, 2003. Introgen undertakes no obligation to publicly release the results of any revisions to any forward-looking statements that reflect events or circumstances arising after the date hereof.
Editor''s Note: For more information on Introgen Therapeutics, Inc., or for a menu of archived press releases, please visit Introgen''s Website at http://www.introgen.com/ .
Contact: Introgen Therapeutics, Inc. C. Channing Burke (512) 708 9310 Ext. 322 Email: c.burke@introgen.com
Introgen Therapeutics, Inc.
© PR Newswire
AUSTIN, Texas (Dow Jones)--Introgen Therapeutics Inc. (INGN) withdrew its secondary offering of 5.5 million common shares, citing market conditions.
Company representatives weren't immediately available to comment further on the withdrawn offer.
The offering was set to price late Thursday via UBS Securities LLC, a unit of UBS AG (UBS). Introgen first announced the offering March 5.
On Dec. 3, the company raised about $20 million by selling 2.86 million shares at $7.00 each. The company planned to use those proceeds for working capital and the development of its anticancer products, Advexin and INGN 241. As of Dec. 31, the company had about $36.4 million in cash and equivalents.
Introgen now has 23.7 million shares outstanding. The stock closed Thursday down 3.8%, or 29 cents, at $7.30 on Nasdaq.
Company Web site: http://www.introgen.com
-Nora Devine; Dow Jones Newswires; 201-938-5400
Order free Annual Report for Introgen Therapeutics Inc.
Visit http://djnewswires.ar.wilink.com/?link=INGN or call 1-888-301-0513
Order free Annual Report for Introgen Therapeutics Inc.
Visit http://djnewswires.ar.wilink.com/?link=INGN or call 1-888-301-0513
Corrected March 19, 200411:40 ET (16:40 GMT)
Dow Jones Newswires
03-19-04 0820ET
Copyright (C) 2004 Dow Jones & Company, Inc. All Rights Reserved.
AUSTIN, Texas, March 23 /PRNewswire-FirstCall/ -- Introgen Therapeutics, Inc. will update its progress in the development of Advexin(R) and INGN 241 at the 95th annual meeting of the American Association of Cancer Research (AACR) held in Orlando, Florida. Advexin currently is in phase 3 trials for the treatment of head and neck cancer and INGN 241 is being evaluated in phase 1 and phase 2 clinical trials for multiple tumor types.
Nine abstracts related to Introgen''s drug candidates will be presented during the conference, which will be held March 27 - March 31, 2004. Two INGN 241 abstracts will be presented on Sunday, March 28; two on Tuesday, March 30 and four on Wednesday, March 31. One Advexin abstract will be presented on Tuesday, March 30.
-- Abstracts 584 and 568 will be presented in poster sessions from 8:00 a.m. to 12:00 p.m. ET on Sunday, March 28. -- Abstract 3778 will be presented in a poster session from 8:00 a.m. to 12:00 p.m. ET on Tuesday, March 30. -- Abstracts LB-331 and 4402 will be presented in poster sessions from 1:00 p.m. to 5:00 p.m. ET on Tuesday, March 30. -- Abstracts 5303, 5135, 4970 and 4891 will be presented in poster sessions from 8:00 a.m. to 12:00 p.m. ET on Wednesday, March 31.
All publications may be accessed online at http://www.aacr.org/ . The presentations will not be web cast.
Introgen is a leading developer of biopharmaceutical products designed to induce therapeutic protein expression using non-integrating gene agents for the treatment of cancer and other diseases. Introgen maintains integrated research, development, manufacturing, clinical and regulatory departments and operates a commercial-scale, CGMP manufacturing facility.
Certain statements in this press release that are not strictly historical may be "forward-looking" statements, which are based on current expectations and entail various risks and uncertainties. Such forward-looking statements include, but are not limited to, those relating to Introgen''s future success with its clinical development program with Advexin and INGN 241 for cancer and other diseases. There can be no assurance that Introgen will be able to commercially develop gene-based drugs, that necessary regulatory approvals will be obtained or that any clinical trials or studies undertaken will be successful or that the proposed treatments will prove to be safe and/or effective. The actual results may differ from those described in this press release due to risks and uncertainties that exist in Introgen''s operations and business environment, including, but without limitation, Introgen''s stage of product development and the limited experience in the development of gene- based drugs in general, Introgen''s dependence upon proprietary technology and current competition, history of operating losses and accumulated deficits, reliance on collaborative relationships, and uncertainties related to clinical trials, the safety and efficacy of Introgen''s product candidates, the ability to obtain the appropriate regulatory approvals, patent protection and market acceptance, as well as other risks detailed from time to time in Introgen''s filings with the Securities and Exchange Commission. Introgen undertakes no obligation to publicly release the results of any revisions to any forward- looking statements that reflect events or circumstances arising after the date hereof.
Editor''s Note: For more information on Introgen Therapeutics, or for a menu of archived press releases, please visit Introgen''s Website at: http://www.introgen.com/ .
Contact: Introgen Therapeutics, Inc. C. Channing Burke (512) 708 9310 Ext. 322 Email: c.burke@introgen.com
Introgen Therapeutics, Inc.
© PR Newswire
Gruß

Kurzkommentar: positive News am Wochenende sollten weiteren starken Kaufdruck auslösen, was m. M. nach zum Breakout führen wird.
Gruß und schönes WE
ORLANDO, Fla., March 30 /PRNewswire-FirstCall/ -- Introgen Therapeutics today reported combined safety and efficacy results based on three multi-center, multi-national Phase 2 clinical trials of Advexin(R) p53 gene therapy in 217 patients with recurrent, squamous cell carcinoma of the head and neck. These data were presented in a late breaking session at the 95th annual meeting of the American Association of Cancer Research taking place March 27-31, 2004 in Orlando, Florida.
The objective overall response rate of Advexin monotherapy was 10 percent (complete and partial response with greater than 50 percent reduction in tumor size). Tumor growth control (stable disease or better) was achieved in 59 percent of all treated lesions. The primary objective of the trials was to assess tumor growth control and safety following Advexin monotherapy in patients with recurrent head and neck cancer. All patients had failed previous conventional treatment.
"The primary endpoint results from the three Phase 2 trials confirm our previously reported findings regarding the activity and safety of Advexin," stated Robert E. Sobol, M.D., Introgen''s senior vice president of medical and scientific affairs. "This multi-center, multi-national analysis is the first detailed report of the combined data from all of our Phase 2 trials in recurrent head and neck cancer. These results were collected from over 40 clinical sites in the U.S. and Europe. We are encouraged by the response and safety data and the consistency of the findings."
Secondary endpoints of the studies included assessments of patient survival. A difference was observed between patients treated with high doses and low doses of Advexin. Patients treated with higher doses had a statistically significant increase in median survival compared to patients treated with lower doses (243 vs. 119 days). Additionally, the overall median survival was longer in patients who were treated with Advexin followed by chemotherapy in each of the studies: Trial 202 (n=20) 330 days; Trial 201 (n=47) 260 days; Trial 207 (n=29) 246 days.
Advexin therapy was administered by intra-tumoral injections. Advexin treatment-related side effects were generally mild to moderate in nature and included transient injection site pain and fever. All patients had been previously treated with radiation therapy and 59 percent had previous chemotherapy. To date, clinical investigators in North America, Europe and Japan have treated over 500 patients with several thousand doses of Advexin therapy, establishing a large safety database.
"We will continue to monitor the survival data from these Phase 2 uncontrolled, non-randomized studies and will further analyze survival duration as part of our ongoing controlled and randomized Phase 3 clinical trials in recurrent head and neck cancer patients," added Dr. Sobol.
About Advexin
There are two multi-national, multi-site Phase 3 trials of Advexin therapy, currently underway in recurrent squamous cell cancer of the head and neck. Introgen has received FDA Fast Track designation for Advexin therapy and Advexin has been designated as an Orphan Drug for the treatment of head and neck cancer under the Orphan Drug Act.
Advexin has been evaluated in a variety of cancer types and in combination with several standard cancer therapies, including radiation and chemotherapy. Data from several published preclinical and clinical studies have demonstrated the ability of Advexin to safely enhance the anti-cancer effects of radiation and chemotherapy treatment.
Advexin supplies p53 protein in very high concentrations in cancer tissue and selectively kills cancer cells. p53 is a normal constituent of cells and is known as a tumor suppressor because it inhibits the growth of tumor cells. One of the major roles of this protein is to eliminate cancerous cells by recognizing when the cell has been damaged by mutations and stopping cell growth to initiate repair. If the cell is damaged beyond repair, p53 initiates the cell death pathway to prevent the cell from growing out of control.
About Introgen
Introgen is a leading developer of biopharmaceutical products designed to induce therapeutic protein expression using non-integrating gene agents for the treatment of cancer and other diseases. Introgen maintains integrated research, development, manufacturing, clinical and regulatory departments and operates a commercial-scale, CGMP manufacturing facility.
Certain statements in this press release that are not strictly historical may be "forward-looking" statements, which are based on current expectations and entail various risks and uncertainties. Such forward-looking statements include, but are not limited to, those relating to Introgen''s future success with its Advexin clinical development program for cancer and other diseases. There can be no assurance that Introgen will be able to commercially develop gene-based drugs, that necessary regulatory approvals will be obtained or that any clinical trials or studies undertaken will be successful or that the proposed treatments will prove to be safe and/or effective. The actual results may differ from those described in this press release due to risks and uncertainties that exist in Introgen''s operations and business environment, including, but without limitation, Introgen''s stage of product development and the limited experience in the development of gene-based drugs in general, Introgen''s dependence upon proprietary technology and current competition, history of operating losses and accumulated deficits, reliance on collaborative relationships, and uncertainties related to clinical trials, the safety and efficacy of Introgen''s product candidates, the ability to obtain the appropriate regulatory approvals, patent protection and market acceptance, as well as other risks detailed from time to time in Introgen''s filings with the Securities and Exchange Commission. Introgen undertakes no obligation to publicly release the results of any revisions to any forward-looking statements that reflect events or circumstances arising after the date hereof.
For more information on Introgen Therapeutics, or for a menu of archived press releases, please visit Introgen''s Website at: http://www.introgen.com/ .
Contact: Introgen Therapeutics, Inc. C. Channing Burke (512) 965 0907 (Cell) (512) 708 9310 Ext. 322 (Office) Email: c.burke@introgen.com Introgen''s Booth in Exhibit Hall #1335
Introgen Therapeutics, Inc.
© PR Newswire